Frequently Asked Questions

The price of in vitro fertilisation (IVF) can vary and depends on various components, including age, reason for infertility, need for surgery, and any additional treatments or procedures that may be necessary. On average, the cost of a single IVF cycle can range from INR 1.5 lakh to 2 lakh.
The cost of a single intrauterine insemination (IUI) can vary based on the age, reason for infertility, need for surgery. On average, the price of a single IUI cycle can range from INR 5000 to 10,000.
Polycystic Ovary Syndrome (PCOS) affects females of reproductive age. The main characteristics of this hormonal disorder include multiple cysts in the ovaries, irregular menstrual cycles, and high levels of male hormones. PCOS can result in various health issues, including infertility, insulin resistance, and obesity.
The exact reason for PCOS is still unknown, but several factors contribute to its development. Genetic factors, insulin resistance, and hormonal imbalances play a significant role. Certain lifestyle habits, like poor nutrition and lack of exercise, can also contribute to PCOS development.
The PCOS symptoms can differ from person to person, but some common symptoms include irregular menstrual cycles, excessive hair growth (hirsutism), acne, weight gain, and difficulty getting pregnant. Women with PCOS may also experience mood swings, depression, and anxiety.
A fertility professional will typically conduct a physical examination to diagnose PCOS and ask questions about your medical history. They may also perform blood analysis to measure hormone levels and execute an ultrasound to check for cysts on the ovaries. The diagnosis of PCOS is based on the presence of specific criteria, including irregular menstrual cycles, elevated male hormone levels, and the presence of cysts on the ovaries.
While there is no cure for PCOS, various treatment modalities can help manage its symptoms. Maintaining a healthy, nutrition-rich meal and regular exercise can help improve symptoms. Medications, such as hormonal contraceptives and insulin-sensitising drugs, may also be prescribed to aid in regulating menstrual cycles and manage other symptoms.
PCOS can lead to several complications if left untreated. Women with PCOS have an increased possibility of type 2 diabetes mellitus, hypertension, and cardiovascular diseases. PCOS can also cause infertility and increase the risk of miscarriage.
Yes, medication can be used to manage the symptoms of PCOS. Hormonal contraceptives, such as contraceptive pills, can help regulate menstrual cycles and diminish symptoms such as acne and excessive hair growth. Insulin-sensitising drugs, such as metformin, can help manage insulin resistance and enhance fertility in women with PCOS.
Making lifestyle adjustments can help manage the signs and symptoms of PCOS. Maintaining a balanced meal that is low in sugar and processed edibles can help maintain insulin levels and promote weight loss. Additionally, focusing on organic fruits, vegetables, and whole grain food products is beneficial. Regular moderate-intensity exercise is crucial in managing PCOS symptoms and maintaining a healthy weight.
PCOS is one of the leading causes of women’s infertility. The hormonal irregularities and irregular menstrual periods associated with PCOS can make it challenging for women to conceive. However, with proper treatment and lifestyle adjustments, many women with PCOS can achieve pregnancy and have a healthy baby.
Yes, PCOS is associated with an increased possibility of developing other health conditions. Insulin resistance associated with PCOS can increase the risk of developing type 2 diabetes. PCOS is also linked to cardiovascular diseases, such as heart disease and stroke, as well as endometrial cancer due to irregular menstruation and hormonal imbalances. PCOS also increases the risk of sleep apnea.
Yes, PCOS symptoms can change over time. Some women may experience more severe symptoms during their teenage years, while others may notice changes in symptoms as they get older. Hormonal fluctuations and lifestyle factors can also influence the severity and frequency of PCOS symptoms.
The Percutaneous Epididymal Sperm Aspiration (PESA) procedure helps retrieve sperm from the epididymis, a structure present at the back of the testicles, in cases where a blockage in the reproductive tract prevents the release of sperm during ejaculation.
PESA is recommended when there is a blockage in the reproductive tract that prevents the release of sperm during ejaculation. Various conditions can cause this prevention, including previous surgeries, congenital abnormalities, or infections. PESA is often performed in conjunction with ICSI to facilitate fertilisation in cases of obstructive azoospermia.
The PESA procedure involves the insertion of a fine needle directly into the epididymis to aspirate sperm. This procedure is typically conducted under local anaesthesia and can be done on an outpatient basis. The fertility experts use these retrieved sperm or ICSI or cryopreserved for future use. PESA is a relatively quick and straightforward procedure that can effectively treat couples with obstructive azoospermia.
As with any medical procedure, PESA carries certain risks and potential complications. These can include bleeding, infection, pain, and swelling at the site of the needle aspiration. In rare cases, damage to the epididymis or surrounding structures may occur. It is essential to discuss the potential risks and complications with your fertility specialist before undergoing the PESA procedure.
The success rates of using sperm retrieved through PESA can vary depending on various factors, including the underlying cause of male infertility and the couples overall health. On average, the success rates of using PESA-retrieved sperm in conjunction with ICSI range from 30% to 40%. Consulting with your fertility specialist to understand your chances of success with PESA is essential.
Testicular Sperm Aspiration (TESA) is a fertility procedure used to retrieve sperm from the testicles in cases where there is no sperm present in the ejaculate. Fertility experts perform this minimally invasive procedure under local anaesthesia, and it is a first-line approach in cases of male infertility.
Testicular Sperm Aspiration (TESA) is recommended when there is a complete absence of sperm in the ejaculate, a condition known as azoospermia. Various factors can result in this condition, including blockages in the reproductive pathway or a lack of sperm production. TESA is also favourable in cases where previous attempts at sperm retrieval, such as PESA or TESE, have been unsuccessful.
The TESA procedure involves the use of a fine needle to aspirate sperm directly from the testicles. This method is generally performed under local anaesthesia to minimise discomfort. After retrieving the sperm, fertility experts can use it for ICSI or cryopreserve it for future use. TESA is a relatively quick and straightforward procedure performed on an outpatient basis.
As with any medical procedure, TESA carries certain risks and potential complications. These can include bleeding, infection, pain, and swelling at the site of the needle aspiration. In rare cases, damage to the testicular tissue or surrounding structures may occur. Therefore, it is necessary to discuss the potential complications with your fertility specialist before undergoing the TESA procedure.
The success rates of using sperm retrieved through TESA can vary depending on various factors, including the underlying reason for male infertility and the couples overall health. On average, the success rates of using TESA-retrieved sperm in conjunction with ICSI range from 30% to 40%. It is essential to counsel your fertility specialist to understand your chances of success with TESA.
Testicular Sperm Extraction (TESE) is a fertility procedure used to directly retrieve sperm from the testicles in cases where there is no sperm present in the ejaculate. It is a more invasive technique than TESA and is commonly used in cases of non-obstructive azoospermia.
Fertility experts recommend the TESE procedure when there is a complete absence of sperm in the ejaculate due to non-obstructive azoospermia. This condition can develop due to various causes, including a lack of sperm production or testicular dysfunction. TESE is often performed in conjunction with ICSI to facilitate fertilisation in cases of non-obstructive azoospermia.
The TESE procedure involves surgically extracting small tissue samples from the testicles. These tissue samples are then carefully dissected and examined under a microscope to recognise and retrieve individual sperm. This procedure is typically conducted under general anaesthesia and may require a short hospital stay. The retrieved sperm is then used for ICSI or cryopreserved for future use.
As with any surgical procedure, TESE carries certain risks and potential complications. These can include bleeding, infection, pain, and swelling at the surgery site. In rare cases, damage to the testicular tissue or surrounding structures may occur. It is crucial to discuss these potential risks and complications with your fertility specialist before undergoing the TESE procedure.
The success rates of using sperm retrieved through TESE can vary and depend on various factors, including the underlying cause of male infertility and the overall health of the couple. On average, the success rates of using TESE-retrieved sperm in conjunction with ICSI range from 30% to 40%. It is essential to seek guidance from your fertility expert to understand your chances of success with TESE.
Several factors are considered when evaluating a male partner’s fertility. A healthcare professional takes a thorough medical history, including any previous surgeries, infections, or medical conditions that may affect fertility. They may also conduct physical examinations to check for any abnormalities or signs of underlying issues. Additionally, a semen analysis is performed to assess sperm quality, count, and motility.
Sperm disorders refer to abnormalities or issues with sperm that can affect fertility. These include azoospermia, characterised by the absence of sperm in the ejaculate, oligospermia, denoting a low sperm count below the normal range, asthenospermia, reflecting poor sperm motility hindering effective fertilisation, and teratospermia, indicating abnormal sperm morphology that may impede fertilisation. Various factors, including hormonal imbalances, genetic abnormalities, infections, lifestyle choices, or certain medical conditions, can cause sperm disorders.
A healthy sperm count is typically regarded to be at least 15 million sperm/millilitre of semen. However, it’s important to note that semen quality can vary, and a low sperm count does not necessarily indicate infertility. Other components, such as sperm motility and morphology, also significantly affect fertility.
Sexually transmitted diseases (STDs) can potentially result in a low sperm count. Infections like chlamydia, gonorrhoea, or syphilis can cause inflammation and injury to the reproductive organs, including the testes and epididymis. These conditions can result in decreased sperm production and quality. It’s crucial to practice safe sex and seek timely treatment for any STDs to prevent long-term effects on fertility.
Excessive alcohol consumption can negatively influence sperm count and quality. Alcohol can disrupt hormone production, affect sperm development, and impair sperm motility. To optimise fertility, limiting alcohol intake or abstaining entirely is advisable.
Managing a healthy lifestyle that involves regular physical activities and a balanced diet can positively influence sperm count and overall fertility. Regular physical activity promotes circulation and hormone balance, while a nutritious meal provides essential nutrients for sperm production. Maintaining a healthy weight and incorporating foods rich in vitamins, antioxidants, and minerals is vital for optimal sperm health.
Hysteroscopy can provide valuable information about female fertility issues by directly visualising the inside of the uterus. It can help diagnose and evaluate various conditions, such as uterine polyps, fibroids, adhesions, and structural abnormalities. Hysteroscopy can also help with certain fertility treatments, such as removing polyps or fibroids, correcting abnormalities, or performing endometrial biopsies. At Ferty9 Fertility Centre, our skilled reproductive specialists utilise hysteroscopy to gather essential information to guide your fertility treatment plan effectively.
Hysteroscopy is a minimally invasive surgical method that allows visualisation and evaluation of the inside of the uterus. It involves inserting a hysteroscope (a thin, flexible catheter with a camera and a light source) through the vagina and cervix into the uterus. At Ferty9 Fertility Centre, our expert surgeons commonly use hysteroscopy to evaluate fertility ailments and perform various treatment modalities.
As with any other medical procedure, hysteroscopy has some potential risks and complications. These may include infection, bleeding, injury to the uterus or surrounding organs, and adverse reactions to anaesthesia. However, complications are rare, and our experienced reproductive specialists at Ferty9 Fertility Centre take every precaution to minimise the risks and provide optimal care.
Surgeons generally perform hysteroscopy on an outpatient basis, using either local or general anaesthesia. They insert a hysteroscope through the vagina and cervix into the uterus and use carbon dioxide gas or saline solution to expand the uterine cavity for better visualisation. The hyst-when is hysteroscopy recommended hysteroscope transmits images to a monitor, allowing the doctor to inspect the uterine lining and identify any abnormalities. The surgeon may use additional instruments to remove polyps, fibroids, or adhesions if necessary. After the final evaluation or treatment completion, the surgeon removes the hysteroscope and finishes the procedure. At Ferty9 Fertility Centre, our skilled reproductive specialists ensure a safe and comfortable hysteroscopic procedure for infertility diagnosis.
Healthcare professionals may recommend hysteroscopy fertility evaluation in various situations. It is instrumental in diagnosing and treating conditions that affect the uterine cavity, such as uterine polyps, fibroids, adhesions, and structural abnormalities. Hysteroscopy can also help remove polyps or fibroids, correct abnormalities, or perform endometrial biopsies. Our experienced reproductive specialists at Ferty9 Fertility Centre will determine if hysteroscopy is necessary in your specific case to provide an accurate diagnosis and appropriate treatment.
Infertility is a disease that affects a couple’s ability to conceive a child. It is described as the inability to achieve pregnancy after one year of routinely unprotected sexual intercourse for women under 35 or after six months for women over 35. Various factors, such as problems with ovulation, sperm quality, or reproductive organs, can cause infertility. It is essential to seek medical help if you suspect you may be infertile, as there are treatments available to help you achieve your dream of starting a family.
At Ferty9 Fertility Centre, we understand that accessibility can be a concern for patients living in rural areas. We strive to make the process as convenient as possible, and we offer the option for you to have your tests done at a nearby clinic. Our team will work closely with your local healthcare provider to ensure that all necessary tests are conducted accurately and efficiently. We strongly believe that everyone, regardless of their geographic location, should have equal access to exceptional fertility services.
Various factors can cause secondary infertility, including an age-related decline in fertility, changes in reproductive health, hormonal imbalances, or previous medical conditions or procedures that may have affected fertility. In some cases, underlying health ailments such as polycystic ovary syndrome (PCOS) or endometriosis can contribute to secondary infertility. Lifestyle habits, such as obesity, cigarette smoking, or excessive alcohol intake, can also play a role. It is essential to consult with a fertility professional to identify the specific causes of secondary infertility and explore treatment options.
Several lifestyle factors can contribute to infertility. These include smoking, excessive alcohol consumption, drug use, poor nutrition, obesity, and exposure to environmental toxins. These lifestyle factors can negatively impact reproductive health in both men and women, affecting sperm quality, hormone levels, and overall fertility. Making some positive lifestyle changes, such as quitting smoking, reducing alcohol consumption, maintaining a healthy weight, and following a balanced diet, can significantly improve fertility outcomes. It is essential to address these lifestyle factors when seeking infertility treatment.
Yes, stress and mental health can have a significant impact on fertility. High levels of stress and anxiety can disturb hormonal balance and intervene with ovulation in women. In men, stress can impact sperm production and quality. Additionally, conditions such as depression and anxiety can impact sexual function and desire, further affecting fertility. It is vital to prioritise mental well-being and manage stress when trying to conceive. Techniques such as counselling, mindfulness, and stress-reducing activities can help improve fertility outcomes.
Lying down after an IUI procedure is not compulsory but is often recommended for a short period. Some women choose to lie down for 10 to 15 minutes to allow the sperm to swim towards the fallopian tubes. However, research on the optimal position after IUI is inconclusive, and the decision to lie down or resume normal activities is a personal choice. It is advisable to discuss this with your fertility expert, who can provide guidance based on your specific circumstances.
While it is essential to take care of yourself after the IUI procedure, there is no need to restrict your activities overly. Taking it easy and avoiding rigorous physical activities or heavy lifting is generally advised for the rest of the day. However, you can resume your routine activities the next day as long as they are not excessively strenuous. Listening to your body and prioritising self-care during this time is crucial.
The number of IUI cycles recommended before considering other options depends on individual circumstances and the advice of your fertility specialist. In general, fertility experts suggest trying IUI for three to six cycles before exploring alternative options such as IVF. However, this may vary based on numerous factors, such as age, cause of infertility, and previous treatment history. Your fertility specialist will assess your progress and guide you on the optimal treatment plan.
After the IUI procedure, most women can resume their normal activities without any restrictions. However, it is advisable to take it easy for the rest of the day. Avoid strenuous physical activities or heavy lifting that could affect the success of the procedure. It is also essential to follow any specific guidelines or instructions provided by your fertility specialist for optimal results.
Intercourse is generally allowed during IUI treatment, but it is essential to follow the guidance of your fertility specialist. In some cases, your fertility specialist may advise abstaining from intercourse for a specific period leading up to and following the procedure to optimise the chances of success. It is crucial to have open communication with your fertility specialist and follow their recommendations for the best possible outcome.
During an IUI cycle, regular monitoring is necessary to track the progress of follicle development and determine the optimal time for the procedure. This monitoring typically involves ultrasound scans and hormone level tests. The number of clinic visits will depend on your individual treatment plan and how your body responds to ovarian stimulation. Rest assured, your fertility specialist will create a tailored monitoring schedule that takes into account your unique circumstances and aims to ensure the best possible outcome.
Abstaining from intercourse before providing a semen sample for IUI is recommended to ensure the best quality and quantity of sperm. Typically, it is advised to abstain from ejaculation for two to five days before the scheduled IUI procedure. However, it is essential to consult with your fertility specialist for specific recommendations based on your unique circumstances.
Timing is crucial for the success of IUI. The procedure is usually performed around ovulation time, typically 24 to 36 hours after the surge in luteinising hormone (LH). Your fertility specialist will closely monitor your menstrual cycle, perform ultrasound scans, and conduct hormone level tests to discover the optimal timing for the procedure. This careful timing enhances the chances of successful fertilisation.
The IUI procedure is generally well-tolerated and relatively painless. It is similar to a regular pelvic exam and does not require anaesthesia. Some women may notice mild discomfort or cramping during the procedure, but it usually subsides quickly. If you have concerns about pain or discomfort, it is essential to discuss them with your fertility specialist, who can provide guidance and address your individual needs.
Ovarian stimulation is a crucial step in an IVF cycle. It involves using fertility medicines to stimulate the ovaries to produce a few mature eggs. The professionals administer fertility medications through injections, and the dosage is carefully monitored based on the individual’s response. Regular ultrasound scans can track follicle growth and determine the optimal time for egg retrieval.
Ovarian hyperstimulation is a potential side effect of ovarian stimulation during an IVF cycle. It occurs when the ovaries respond excessively to fertility medications, leading to the development of a large number of follicles. It can result in ovarian enlargement and fluid accumulation in the abdomen. Severe cases of ovarian hyperstimulation syndrome (OHSS) are rare but can cause discomfort and require medical intervention. Your fertility expert will closely monitor your response to medications and take appropriate measures to minimise the risk of hyperstimulation.
Egg retrieval is a minor surgical procedure usually performed under sedation or anaesthesia. Using ultrasound guidance, fertility experts insert a thin needle through the vaginal wall and reach the ovaries and aspirate the mature eggs from the follicles. The retrieved eggs are then handed to the laboratory for further processing and fertilisation.
The number of eggs or ova required for a successful IVF cycle differs depending on individual circumstances. However, the quality of eggs is equally essential. Your fertility specialist will aim to retrieve an optimal number of mature eggs based on your age, ovarian reserve, and previous treatment history.
After the eggs are retrieved, embryologists carefully examine them in the laboratory. The eggs are then prepared for fertilisation by removing the surrounding cells. For conventional IVF, the eggs are either placed in a culture dish along with a sample of sperm or subjected to intracytoplasmic sperm injection (ICSI) for cases with severe male factor infertility or previous fertilisation issues.
We usually put a maximum of two embryos into the woman’s uterus during one cycle. If there are more embryos left after the first cycle, we cryopreserve them for later. This way, if the first try doesn’t work, we can use the frozen embryos in another attempt.
Embryo transfer is the final step of the IVF process, where the selected embryos are transferred into the woman’s uterus. It is a relatively simple and painless procedure that does not require anaesthesia. Using a catheter, fertility experts gently transfer the embryos into the uterus with the guidance of ultrasound imaging. The number of embryos transferred depends on various factors, including the woman’s age, embryo quality, and preferences.
One of the most common questions when undergoing in vitro fertilisation (IVF) is the number of embryos that should be transferred. The number of embryos transferred can differ depending on several components, including the woman’s age and the quality of the embryos. Generally, transferring a maximum of two embryos is recommended to reduce the possibility of multiple pregnancies.
After the embryo transfer step, following certain dos and don’ts is essential to optimise the possibilities of implantation and a successful pregnancy. Some of the dos include taking prescribed medications on time, maintaining a healthy lifestyle, eating a balanced diet, and getting adequate rest. On the other hand, some of the don’ts include avoiding strenuous activities, refraining from smoking and alcohol intake, and avoiding certain medicines that may interfere with implantation. It is crucial to follow the instructions given by the fertility centre to increase the likelihood of a positive outcome.
Rest is an essential aspect of the post-embryo transfer period. While it is recommended to take it easy and rest for a few days after the procedure, no specific duration applies to everyone. The doctor will provide guidance based on individual circumstances. Balancing rest and light physical activity is essential to ensure optimal blood flow to the uterus while avoiding excessive strain. Following the doctor’s advice and listening to one’s body is vital during this critical period.
It is possible to achieve pregnancy with frozen embryos. Cryopreservation, the process of freezing embryos, has revolutionised the field of assisted reproductive technology. Frozen embryos can be stored for future use, allowing couples to undergo additional IVF cycles without the need for further ovarian stimulation and egg retrieval. The success rates with frozen embryos are comparable to fresh embryos and, in some cases, may even be higher. Ferty9 Fertility Centre offers comprehensive frozen embryo transfer services, allowing couples to achieve pregnancy when the time is right for them.
The possibility of birth defects in babies conceived through IVF is generally similar to that of babies conceived naturally. However, it is essential to note that certain factors, such as the woman’s age and any underlying genetic conditions, can influence the risk. The team at Ferty9 Fertility Centre identifies potential complications and provide appropriate guidance. It is essential to have open and honest discussions with medical experts to address any concerns and make informed decisions.
The possibilities of giving birth to twins or multiple births are higher with IVF compared to natural conception. It is primarily due to the practice of transferring multiple embryos to increase the likelihood of success. While the idea of twins may be exciting for some couples, it is essential to consider the potential complications and challenges associated with multiple pregnancies. The fertility specialists at Ferty9 Fertility Centre closely monitor the number of embryos transferred to minimise the chance of multiple pregnancies while maximising the chances of a successful single pregnancy.
If either the husband or the wife gets sick during an IVF cycle, it is crucial to inform the fertility centre immediately. The medical team will guide on whether to continue or postpone the cycle based on the specific circumstances. Sometimes, it may be necessary to delay the cycle to ensure the best possible outcome. Communication with the fertility centre ensures proper care and support during the IVF cycle.
IVF (in vitro fertilisation) and ICSI (intracytoplasmic sperm injection) are two different techniques used in assisted reproductive technology. IVF includes the fertilisation of eggs and sperm outside the body, followed by the transfer of resulting embryos into the uterus. ICSI, on the other hand, is a specialised form of IVF that involves the direct injection of a single sperm into each egg. ICSI is typically recommended in cases of male factor infertility, where the sperm may have difficulty fertilising the egg naturally. The fertility specialists at Ferty9 Fertility Centre will recommend the most suitable procedure based on individual circumstances.
Coverage for infertility treatment varies based on the health insurance provider and the specific policy. Some health insurance services may offer partial or complete coverage for fertility treatments such as IVF, while others may not provide any coverage at all. It is essential to review the insurance policy and discuss it with the insurance provider to understand the extent of coverage for infertility treatment. Ferty9 Fertility Centre also provides guidance and assistance in navigating the financial aspects of fertility treatment.
If the first IVF cycle is unsuccessful, several options exist for subsequent attempts. The fertility specialist will evaluate the reasons for the failure of the previous cycle and recommend appropriate adjustments for the next cycle. These may include changes in medication protocols, additional testing, or considering alternative approaches such as frozen embryo transfer or using donor eggs or sperm. The team at Ferty9 Fertility Centre will provide personalised recommendations based on individual circumstances, aiming to optimize the chances of success in subsequent attempts.
The quality of eggs and sperm plays a vital role in the success of IVF. Higher-quality eggs and sperm have a higher chance of successful fertilisation and implantation. Factors such as age, lifestyle habits, and underlying medical conditions can impact the quality of eggs and sperm. While it is impossible to change one’s age, certain measures can improve the overall quality. Maintaining a healthy lifestyle, including regular physical exercise, eating a balanced meal, and avoiding smoking and excessive alcohol consumption, can improve egg and sperm quality. Additionally, the fertility specialist may prescribe certain medications and supplements to enhance the quality of eggs and sperm.
Micro TESE, or Microsurgical Testicular Sperm Extraction, is a specialised technique for retrieving sperm in cases of non-obstructive azoospermia. It involves using an operating microscope to identify and extract individual sperm from the testicles with greater precision and accuracy.
The fertility experts generally recommend the Micro TESE procedure in cases of non-obstructive azoospermia where there is a complete absence of sperm in the ejaculate. Various factors, including a lack of sperm production or testicular dysfunction, can cause this condition. Micro TESE is often performed in conjunction with ICSI to facilitate fertilisation in cases of non-obstructive azoospermia.
The main difference between Micro TESE and traditional TESE lies in the level of precision and accuracy in sperm retrieval. Micro TESE utilises an operating microscope to identify and extract individual sperm from the testicles, whereas traditional TESE relies on macroscopic examination of the tissue samples. Using an operating microscope in Micro TESE allows for better visualisation of the testicular tissue, increasing the chances of finding viable sperm.
As with any surgical procedure, micro TESE carries certain risks and potential complications. These can include bleeding, infection, pain, and swelling at the site of the surgery. In rare cases, damage to the testicular tissue or surrounding structures may occur. It is essential to discuss the potential risks and complications with your fertility specialist before undergoing the Micro TESE procedure.
The success rates of using sperm retrieved through Micro TESE can vary depending on various factors, including the underlying cause of male infertility and the overall health of the couple. On average, the success rates of using Micro TESE-retrieved sperm in conjunction with ICSI range from 30% to 40%. Consulting with your fertility specialist to understand your chances of success with Micro TESE is essential.
Laparoscopy is a minimally invasive surgical approach enabling healthcare providers to visualise and assess reproductive organs, including the uterus, fallopian tubes, and ovaries. It involves making small incisions in the abdomen and inserting a laparoscope, a thin, lighted tube with a camera, to examine the pelvic structures. At Ferty9 Fertility Centre, we use laparoscopy procedures for fertility evaluation and treatment.
Fertility professionals may recommend laparoscopy for fertility evaluation in various situations. It is beneficial in diagnosing conditions such as endometriosis, pelvic adhesions, and ovarian cysts, which can impact fertility. Laparoscopy can also aid in removing adhesions, treating endometriosis, or repairing structural abnormalities hindering conception. Our skilled surgeons at Ferty9 Fertility Centre will determine if laparoscopy is necessary in your specific case to provide an accurate diagnosis and appropriate treatment.
Healthcare professionals typically perform laparoscopy under general anaesthesia. They make small incisions in the abdomen and insert a laparoscope to visualise the pelvic structures. They use carbon dioxide gas to inflate the abdomen, creating space for improved visualisation. Additional instruments may be inserted through the incisions to perform necessary procedures, such as removing adhesions or cysts. Once the evaluation or treatment is complete, the surgeon pulls out instruments and closes the incisions with sutures or adhesive strips. At Ferty9 Fertility Centre, our experienced surgical team ensures a safe and comfortable laparoscopic procedure for infertility diagnosis.
As with any surgical procedure, laparoscopy has some potential risks and complications. These may include bleeding, infection, damage to surrounding structures or blood vessels, and adverse reactions to anaesthesia. However, complications are rare, and our skilled surgeons at Ferty9 Fertility Centre take every precaution to minimise the risks and provide optimal care.
Laparoscopy can provide valuable information about female fertility problems by directly visualising the reproductive organs. It can help diagnose and evaluate conditions such as endometriosis, pelvic adhesions, ovarian cysts, and structural abnormalities. Laparoscopy can also help implicate certain fertility treatments, such as removing adhesions, treating endometriosis, or repairing fallopian tubes. At Ferty9 Fertility Centre, our skilled surgeons utilise laparoscopy to gather essential information to effectively guide your fertility treatment plan.
Laparoscopy can improve fertility by diagnosing and treating conditions like endometriosis or tubal disease. For some, it might be a step towards achieving pregnancy, either naturally or with assisted reproductive technologies.
Endometrial polyps are outgrowths in the lining of the uterus (endometrium). They are usually non-cancerous and can vary in size from a few millimetres to several centimetres. Though the precise cause remains uncertain, hormonal imbalances, inflammation, or alterations in the endometrium’s reaction to estrogen may play a role in their development. Endometrial polyps may remain asymptomatic or cause irregular bleeding, infertility, and an increased risk of miscarriage.
Endometrial polyps can create an unfavourable environment for implantation and increase the risk of miscarriage. They may interfere with the implantation process by altering the endometrial lining’s receptivity, reducing the embryo’s ability to attach and proliferate. Polyps can also lead to irregular uterine bleeding, which may affect the timing of ovulation and intercourse, reducing the chances of conception. Sometimes, larger polyps may physically obstruct the fallopian tubes or cervix, hindering sperm transport or embryo passage. Removing the polyps through a procedure called hysteroscopy can improve fertility outcomes.
The exact reason for endometrial polyps is unknown, but hormonal imbalances, such as elevated estrogen levels, are believed to play a role in their development. Chronic uterine inflammation, possibly due to infections or other underlying conditions, can also play a role. Other factors contributing to developing endometrial polyps include obesity, certain medications, and obesity.
Some women with endometrial polyps may not experience any symptoms. However, common symptoms include irregular or heavy menstrual bleeding, bleeding between periods, and pelvic pain. Women with endometrial polyps may also experience infertility or recurrent miscarriages due to disruptions in the uterine environment. These symptoms can overlap with other gynaecological conditions, so a comprehensive evaluation by a healthcare professional is necessary for further evaluation.
Diagnosing endometrial polyps involves a comprehensive analysis of medical history, physical examination, and diagnostic imaging. Transvaginal ultrasound can help visualise the uterine cavity and detect polyps. Hysteroscopy, where a thin, flexible catheter with a camera is inserted into the uterus, also helps visualise the polyps. Sometimes, imaging modalities such as saline infusion sonohysterography (SIS) or hysterosalpingography (HSG) may be employed to enhance visualisation of the endometrial lining. Healthcare professionals may also perform a biopsy to rule out the presence of cancerous cells.
The treatment options for endometrial polyps depend on their size, number, and symptoms. In some cases, if the polyps are small and asymptomatic, no treatment may be necessary. However, for symptomatic polyps or those affecting fertility, surgical removal through hysteroscopy or surgical resection is typically recommended. Regular follow-up evaluations are essential to monitor for recurrence and ensure optimal uterine health, particularly in women with risk factors or ongoing symptoms.
Endometriosis is a condition in which the tissue that usually lines the inside of the uterus, called the endometrium, grows outside the uterus. This displaced tissue can be found on the ovaries, fallopian tubes, or other pelvic organs, leading to inflammation, scarring, and potential fertility issues.
The exact reason for endometriosis is unknown, but several theories exist. Retrograde menstruation, in which menstrual blood flows back into the pelvis instead of out of the body, is one possible cause. Hormonal imbalances, immune system dysfunction, and genetic factors may also contribute to the development of endometriosis.
Common symptoms of endometriosis include pelvic pain, painful menstrual periods, pain during intercourse, and infertility. Some women may also experience heavy or irregular menstrual bleeding, fatigue, or gastrointestinal symptoms such as constipation or diarrhoea.
The diagnosis of endometriosis includes a combination of medical history, physical examination, and diagnostic procedures. These may include pelvic ultrasound, magnetic resonance imaging (MRI), or laparoscopy, which allows direct visualisation and removal of endometrial tissue.
Endometriosis can indeed lead to infertility. The condition refers to endometrial tissue outside the uterus, which can cause inflammation, scarring, and adhesions that interfere with normal reproductive functions. Endometriosis can affect ovulation, egg quality, fallopian tube function, and implantation, making it more challenging to conceive.
While there is no known cure for endometriosis, various treatment modalities are available to manage symptoms and improve fertility. These can include pain management strategies, hormone therapy, or surgical interventions to remove endometrial tissue or correct any anatomical abnormalities.
Various treatment approaches can help manage endometriosis-related pain. Over-the-counter pain relievers may provide temporary relief, while hormonal drugs such as contraceptive pills or GnRH agonists can help control symptoms. In severe cases, fertility professionals may recommend surgery to remove endometrial tissue or address any structural abnormalities.
Pregnancy can provide temporary relief from endometriosis symptoms for some women. The hormonal alterations that occur during pregnancy can suppress the growth of endometrial tissue. However, it’s important to note that symptoms may return after childbirth or once hormonal balance is restored.
Certain lifestyle changes can help manage endometriosis symptoms. Eating a nutrient-rich diet, exercising regularly, managing stress and anxiety levels, and getting adequate sleep can all contribute to overall health and potentially alleviate some symptoms.
Endometriosis can have long-term effects on a woman’s reproductive health. It can cause scarring and adhesions, leading to chronic pain and potential fertility issues. Additionally, endometrial tissue growth may continue over time, causing progressive symptoms and impacting quality of life.
There is a potential link between endometriosis and certain cancers, particularly ovarian cancer. Women with endometriosis may have a higher chance of developing ovarian cancer, although the overall risk is still relatively low. Regular check-ups and open communication with healthcare providers are essential to monitor potential risks.
Endometriosis can indeed recur after treatment or surgery. The recurrence rate can vary and depends on various components, including the disease’s severity, the treatment’s effectiveness, and individual factors. Close monitoring and ongoing management are essential to promptly address any recurring symptoms.
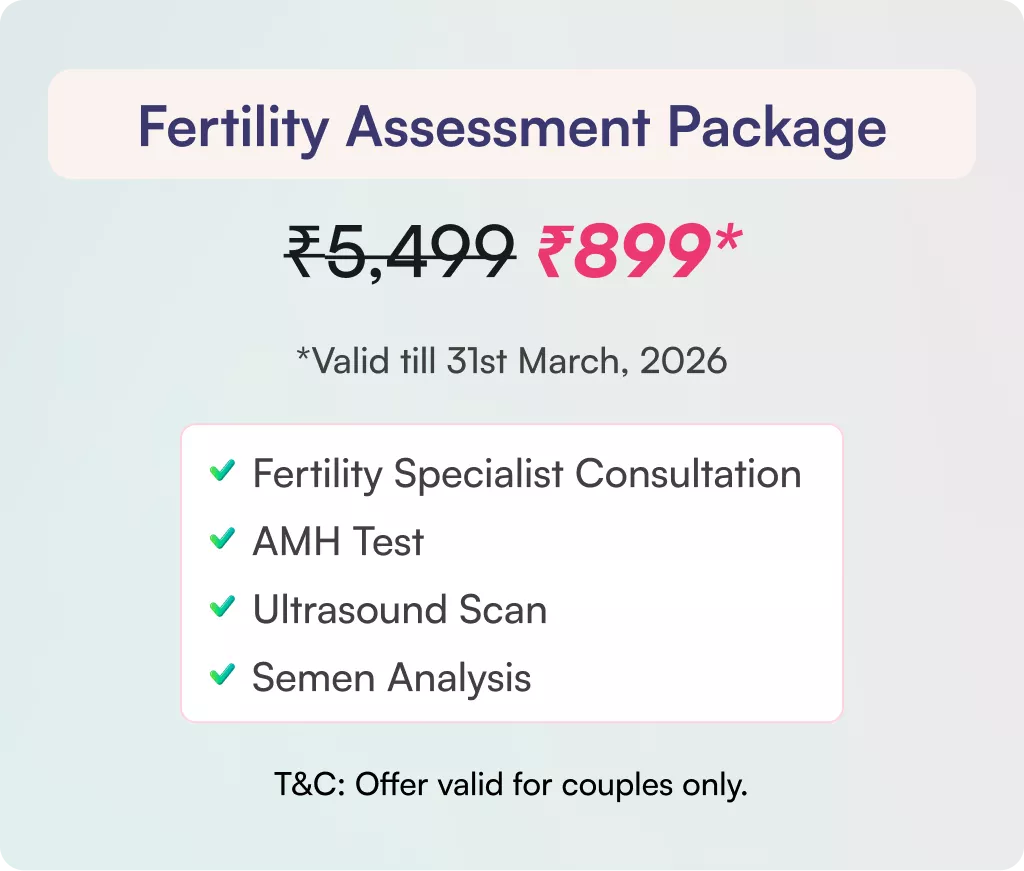
The cost of different fertility services can vary based on the specific services and the location of the fertility centre. It is best to consult with a fertility specialist to get an accurate estimate of the costs associated with the services you may need.
Infertility testing is quite simple, and the cost can vary depending on the specific tests required and the location of the fertility centre. On average, it can range from INR 1000 to INR 5000.
IVF is considered an expensive treatment due to the complex procedures and laboratory techniques involved. The cost of IVF includes ovarian stimulation medications, egg retrieval procedures, laboratory fees for fertilisation and embryo culture, and embryo transfer procedures.
In addition to the cost of the fertility treatment itself, there may be additional expenses to consider. These can include medications, consultations with fertility specialists, additional diagnostic tests, genetic testing, and any necessary surgical procedures.
Insurance coverage for infertility treatments varies based on the specific policy and insurance provider. Some insurance plans offer coverage for infertility treatments, while others may have limited or no coverage. It is best to check with your coverage provider to determine your available coverage.
The coverage of egg freezing by insurance depends on the insurance provider and the policy you have taken. Some insurance plans offer coverage for fertility preservation, including egg freezing, while others may have limited or no coverage. It is best to check with your insurer to determine available coverage.
At Ferty9 Fertility Centre, we aim to make fertility treatments accessible to as many couples as possible. We accept various insurance plans to meet the diverse needs of our patients. However, it’s essential to understand that each insurance plan is unique, and coverage for infertility treatments can vary. We recommend you contact your insurer directly to determine the specifics of your coverage. Our knowledgeable and dedicated team is also available to help you understand your insurance benefits and navigate the process, ensuring you are well informed at every step.
Infertility is a medical condition that affects millions of people and couples worldwide. Many employers recognise the importance of supporting their employees in their journey towards parenthood. While infertility coverage varies from employer to employer, many companies now offer infertility benefits as part of their employee health plans. These resources may include coverage for diagnostic tests, fertility medications, and even assisted reproductive technologies such as in vitro fertilisation (IVF). Reviewing your employee benefits package or consulting with your human resources department to determine the extent of your infertility coverage is advisable.
Egg freezing, or oocyte cryopreservation, is a well-known fertility preservation technique that allows women to preserve their eggs for future use. It can be a valuable option for women who want to delay childbearing due to medical or personal reasons. Similar to infertility coverage, the availability of egg freezing benefits through your employer depends on your company’s specific policies. Some employers may include egg freezing as part of their fertility benefits, while others may not. It is essential to check with your employer or insurance provider to understand the degree of your coverage. At Ferty9 Fertility Center, we offer comprehensive egg freezing services and can guide you through the process of utilising your insurance benefits, if applicable.
Understanding the financial aspect of fertility treatments is crucial for couples who are exploring their options. At Ferty9 Fertility Centre, we offer zero cost EMI option to guarantee our patients can access the care they need without considering undue financial burden. Our dedicated financial counsellors are here to guide you every step of the way. Our goal is to provide transparency and support throughout the financial process so our patients can focus on their journey towards parenthood.
Cryopreservation is a technique used to freeze and store biological materials, such as sperm, eggs, or embryos, for future use. It is an integral component of assisted reproductive technology, offering individuals and couples the opportunity to preserve their fertility when facing factors that may compromise their reproductive capabilities. Cryopreservation has revolutionised the field of reproductive medicine, allowing for the successful preservation and utilisation of gametes and embryos.
Cryopreservation involves the careful freezing of biological materials using specialised techniques and equipment. The process typically includes the addition of cryoprotectants to protect the biological cells from damage during the freezing and thawing process. The samples are then cooled at a controlled rate and stored in liquid nitrogen at extremely low temperatures. This freezing technique effectively preserves the viability and functionality of sperm, eggs, and embryos, enabling their future use in fertility treatments.
Elective cryopreservation refers to the deliberate choice of freezing and storing embryos for future use instead of transferring them immediately during an IVF cycle. This approach provides several advantages over fresh embryo transfer. Frozen embryos have a higher chance of survival and implantation due to the ability to select the most viable embryos through PGT. Additionally, cryopreservation allows for multiple transfer attempts, increasing the overall success rates of IVF treatments. Elective cryopreservation gives individuals and couples greater flexibility in family planning and increases the chances of achieving their desired parenthood goals.
The cryopreservation technique for sperm, eggs, and embryos is a complex process. After collection, the samples undergo a process called vitrification, which involves rapid freezing in a high concentration of cryoprotectants. These cryoprotectants play a crucial role in preventing ice crystal formation, which can damage the biological cells during the freezing process. The samples are then preserved in liquid nitrogen at ultra-low temperatures until they are needed for future use. This cryopreservation technique is essential for the long-term preservation of sperm, eggs, and embryos, maintaining their viability and fertility potential.
Embryos can be safely frozen and stored for extended periods. The duration of cryopreservation depends on various factors, such as the quality of the embryos and the regulations of the fertility clinic. In many cases, one can store embryos for several years without compromising their viability or fertility potential. Fertility clinics typically have stringent protocols in place to ensure the proper storage and monitoring of frozen embryos, providing individuals and couples with the flexibility to plan their family-building journey according to their specific circumstances.
In the frozen embryo transfer (FET) procedure, cryopreserved embryos are thawed and transferred into the uterus during an IVF cycle. FET offers several advantages over fresh embryo transfer, including a higher chance of successful implantation and pregnancy. The ability to select the healthiest embryos through PGT and prepare the uterine lining for optimal receptivity contributes to the increased success rates of FET. This technique allows individuals and couples to maximise their possibilities of achieving a successful pregnancy while minimising the risks associated with multiple embryo transfer.
Preparing for a frozen embryo transfer cycle involves a series of carefully planned steps to optimise the chances of successful implantation and pregnancy. The process typically starts with hormonal therapy to prepare the uterine lining for embryo transfer. This therapy may involve administering estrogen and progesterone to mimic the natural hormonal changes during a natural menstrual cycle. The fertility clinic will closely monitor the progress of the cycle through ultrasound scans and blood tests to ensure that the uterine lining is ready for implantation. Once the optimal conditions are achieved, the surgeon thaws and transfers cryopreserved embryos into the uterus, following which the individual or couple will undergo a period of waiting to determine the outcome of the cycle.
Ovarian hyperstimulation syndrome (OHSS) is a potentially serious aftermath that can occur during fertility treatments, such as in ovarian stimulation for in vitro fertilisation (IVF). In this condition, ovaries respond excessively to fertility medications, causing fluid buildup in the abdominal cavity and, in severe instances, the chest cavity. OHSS symptoms range from mild to severe and can cause discomfort, pain, abdominal manifestations, rapid weight gain, and, in rare cases, life-threatening complications. At Ferty9 Fertility Centre, we follow mild stimulation protocol to prioritise the safety and well-being of our patients and closely monitor the development of OHSS during fertility treatments.
The symptoms of OHSS can vary from mild to severe and may include abdominal bloating, discomfort or pain, nausea, and vomiting. In severe cases, OHSS can cause rapid weight gain, decreased urine output, and shortness of breath. Fluid buildup in the chest can also result in breathing difficulties and blood clotting abnormalities. If you experience any of these symptoms during or after fertility treatment, it is crucial to seek immediate medical attention at Ferty9 Fertility Centre.
The leading cause of OHSS is the excessive use of fertility medications that activate the ovaries to produce multiple eggs. The excess production of eggs can lead to ovarian enlargement and the release of substances that cause blood vessels to seep fluid into the abdomen. Certain risk factors, such as a young age, polycystic ovary syndrome (PCOS), and a high ovarian reserve, may increase the likelihood of developing OHSS. Our experienced fertility specialists at Ferty9 Fertility Centre carefully monitor the response to fertility medications to minimise the risk of OHSS.
OHSS is relatively uncommon, occurring in approximately 1-10% of women undergoing fertility treatments, particularly IVF. However, the risk can vary depending on individual factors and the specific fertility protocol. At Ferty9 Fertility Centre, we employ personalised treatment plans and closely monitor our patients to minimise the risk of OHSS and ensure optimal outcomes.
Certain factors may increase the risk of developing OHSS, including a young age, a high ovarian reserve, polycystic ovary syndrome (PCOS), and a history of OHSS in previous fertility treatments. Additionally, the use of certain fertility medications and a robust response to ovarian stimulation can also increase the likelihood of developing OHSS. At Ferty9 Fertility Centre, our experienced fertility specialists will assess your risk factors and adjust your treatment plan accordingly to minimise the risk of OHSS.
Oncofertility is a multidisciplinary field that focuses on preserving fertility in cancer patients. Cancer treatments like chemotherapy and radiotherapy can damage reproductive organs or disrupt hormonal balance, leading to infertility or premature menopause. At Ferty9 Fertility Centre, we provide specialised oncofertility facilities to help cancer patients preserve their fertility options before undergoing cancer treatment.
Cancer can affect fertility in various ways, depending on the type and location of the cancer, as well as the treatment modalities used. Chemotherapy drugs and radiotherapy can damage the eggs or sperm, disrupt hormone production, and cause irreversible damage to the reproductive organs. At Ferty9 Fertility Centre, we understand the unique challenges faced by cancer patients and provide personalised fertility preservation options.
At Ferty9 Fertility Centre, we offer several oncofertility options to cancer patients, including egg freezing, sperm freezing, and embryo freezing. These modalities allow individuals to preserve their reproductive cells or tissues before undergoing cancer treatment. Our team of experts will guide you through the process and provide the necessary support to make informed choices about your fertility preservation options.
Ovulation is the process by which a mature ovum or egg is released from a female’s ovary and becomes available for fertilisation. It typically occurs once a month, halfway through the menstrual cycle. Ovulation is a crucial event in the reproductive process and is necessary for pregnancy to occur.
Ovulation disorders refer to conditions that interfere with or prevent the regular release of eggs from the ovaries. These disorders include polycystic ovary syndrome (PCOS), premature ovarian failure, and hypothalamic dysfunction. Ovulation disorders can result in irregular (oligoovulation) or absence of ovulation (anovulation), making it difficult for females to conceive naturally. Ovulation disorders can also lead to menstrual irregularities, mood changes, or sudden weight changes.
Ovulation disorders can arise from various causes, including hormonal imbalances, polycystic ovary syndrome (PCOS), thyroid disorders, and premature ovarian failure. Other factors that can contribute to ovulation disorders include excessive exercise, extreme weight loss or gain, certain medications, pituitary gland abnormalities, and chronic illnesses. Identifying the underlying cause of ovulation disorders is crucial for developing targeted treatment strategies to restore ovulatory function and improve fertility.
The primary symptom of ovulation disorders is irregular or absence of menstrual cycles. Women with ovulation disorders may also experience other symptoms, such as excessive hair growth, hot flashes, acne, weight gain or loss, vaginal dryness, and difficulty getting pregnant. If you are experiencing any of these symptoms, seek guidance from a healthcare professional for an appropriate diagnosis and treatment.
A healthcare provider may conduct a physical analysis and ask about your medical history to diagnose ovulation disorders. They may also prescribe other blood tests to measure levels of essential reproductive hormones and an ultrasound to evaluate the condition of the ovaries. Tracking your menstrual cycles and monitoring basal body temperature can also help identify ovulation patterns. Additional tests, like thyroid function tests or pelvic laparoscopy, may help identify underlying conditions contributing to ovulation disorders.
Treatment of ovulation disorder aims to restore regular ovulatory function and improve fertility. The treatment options for ovulation disorders depend on the underlying cause and the individual’s fertility goals. Lifestyle changes can help regulate ovulation. These include managing a healthy weight, balancing exercise regimen, and reducing stress. Doctors may also prescribe medications to stimulate ovulation. In more severe cases, assisted reproductive technologies, such as in vitro fertilisation (IVF), may be recommended.
Tubal disorders refer to conditions that affect the fallopian tubes, which carry the ovum or eggs from the ovaries to the uterus. Tubal disorders can include inflammation, blockages, or scarring of the fallopian tubes, which can intervene with the fertilisation of the egg and prevent pregnancy.
Tubal disorders can significantly impact fertility. If the fallopian tubes are obstructed or damaged, it can hinder the sperm from reaching the egg and prevent the fertilisation process. Additionally, tubal disorders can increase the possibility of ectopic pregnancy, where the fertilised ovum implants outside of the uterus.
Various factors can lead to the development of tubal disorders, including pelvic inflammatory disease (PID), caused by sexually transmitted infections. Other causes of tubal disorders include previous pelvic surgeries like appendectomies or surgeries for ectopic pregnancies, endometriosis, adhesions due to abdominal or pelvic surgeries, and certain genetic conditions. These conditions can lead to blockages, scarring, or damage to the fallopian tubes, hindering fertility.
Tubal disorders may not have any noticeable symptoms, especially in the early stages. However, some women may experience pelvic pain, abnormal vaginal discharge, and irregular menstrual cycles. If you are experiencing any of these symptoms, consulting a fertility professional for a proper diagnosis is crucial.
A healthcare professional asks about your medical history and may perform a physical examination to diagnose tubal disorders. They may also order imaging tests, such as a hysterosalpingogram (HSG) or a laparoscopy, to evaluate the condition of the fallopian tubes and identify any abnormalities. Hysterosalpingography (HSG) involves injecting a dye into the uterus and taking X-rays to visualise the fallopian tubes, which can detect blockages or abnormalities. Transvaginal ultrasound may also reveal tubal issues, such as fluid accumulation in the tubes. Laparoscopy, a minimally invasive surgical procedure, allows direct visualisation of the fallopian tubes and can identify blockages, scarring, or other abnormalities. These diagnostic methods help fertility specialists determine the extent and nature of tubal disorders to guide appropriate treatment decisions.
The treatment options for tubal disorders depend on the severity of the condition and the person’s fertility goals. In some cases, healthcare professionals may recommend surgery to repair or remove any blockages or scar tissue in the fallopian tubes. In more severe cases, in vitro fertilisation (IVF) may be recommended, where the eggs are fertilised outside the body and then transferred to the uterus.
Unexplained infertility is regarded as the absence of a clear cause for a couple’s infertility after a thorough evaluation. Despite extensive tests and examinations, no identifiable issues with sperm count, fallopian tubes, or ovulation are found. Unexplained infertility can be frustrating, but various treatment modalities are available to help couples achieve pregnancy.
The causes of unexplained infertility remain unknown despite extensive testing. However, certain factors, including subtle hormonal imbalances, egg quality issues, sperm function abnormalities, or genetic factors, may contribute to this condition. Psychological stress and lifestyle factors can also play a role.
Unexplained infertility is more common than many people realise, accounting for a significant percentage of infertility cases. It can be a frustrating diagnosis, but it’s important to remember that effective treatments are available, and many couples with unexplained infertility can conceive with medical assistance.
To diagnose unexplained infertility, fertility professionals may conduct a comprehensive analysis, which may include a detailed medical history, physical examination, hormone testing, semen analysis, and imaging studies such as hysterosalpingography (HSG) or ultrasound. They may also recommend additional specialised tests based on individual circumstances.
Couples facing unexplained infertility have several treatment options available. These can include ovulation induction with medication, IUI, or more advanced techniques like IVF. In some cases, healthcare professionals may recommend additional procedures like intracytoplasmic morphologically selected sperm injection (IMSI) or preimplantation genetic testing (PGT) to maximise chances of success.
Uterine fibroids are non-cancerous outgrowths that develop in the muscular wall of the uterus. They can vary in number and size and are common among women of reproductive age. Fibroids are composed of smooth muscle tissue and fibrous connective tissue. Symptoms can include heavy menstrual bleeding, pelvic pain or pressure, frequent urination, and reproductive issues. While most fibroids do not cause fertility problems, certain factors, such as their location and size, can interfere with conception. At Ferty9 Fertility Centre, our experienced reproductive specialists can evaluate the impact of uterine fibroids on fertility and recommend appropriate treatment options.
Uterine fibroids can negatively affect fertility in several ways. Large fibroids can deform the shape of the uterus, making it challenging for a fertilised egg to implant. Fibroids can also obstruct the fallopian tubes or disrupt the normal movement of sperm, hindering fertilisation. Additionally, fibroids can cause heavy or prolonged menstrual bleeding, which can lead to anaemia and decrease the chances of conception. At Ferty9 Fertility Centre, our team of experts will assess the impact of uterine fibroids on your fertility and develop a personalised treatment plan.
The exact reason for uterine fibroids is still unidentified, but several factors contribute to their development. Hormonal fluctuations, specifically an excess of estrogen, can promote the growth of fibroids. Genetic factors, family history, and certain lifestyle factors, such as obesity and a sedentary lifestyle, may also increase the risk. At Ferty9 Fertility Centre, we aim to identify the underlying causes of uterine fibroids to provide effective treatment modalities tailored to your specific needs.
Uterine fibroids can present with various symptoms, including heavy menstrual bleeding, prolonged periods, pelvic pain or pressure, lower back pain, frequent urination, and difficulty emptying the bladder. Some females may also experience pain during sexual intercourse or have an enlarged abdomen. However, not all women with fibroids experience symptoms. If you are experiencing any of these symptoms, it is essential to consult with our experts at Ferty9 Fertility Centre for accurate diagnosis and appropriate management.
At Ferty9 Fertility Centre, we use advanced diagnostic techniques to diagnose uterine fibroids accurately. These may include a pelvic ultrasound, hysteroscopy, or MRI. A pelvic ultrasound can provide detailed images of the uterus and help identify the presence, size, and location of uterine fibroids. Hysteroscopy involves inserting a thin catheter with a camera and a light source into the uterus to visualise any abnormalities. MRI scans can provide a more comprehensive evaluation of the fibroids and their impact on fertility.
The treatment options for uterine fibroids depend on various factors, including the size, number, and position of the fibroids and your fertility goals. At Ferty9 Fertility Centre, we offer a range of treatment options, including medication to manage symptoms and shrink the fibroids, minimally invasive procedures such as uterine artery embolisation or MRI-guided focused ultrasound surgery, and surgical removal of the fibroids. Our dedicated team will work with you to determine the most appropriate treatment plan for your individual needs.
Yes, IVF pregnancies have a slightly higher chance of preterm delivery compared to natural pregnancies. However, this is primarily due to factors associated with IVF rather than the procedure itself:
- Higher likelihood of multiple pregnancies (twins or triplets)
- Advanced maternal age in many IVF patients
- Underlying fertility conditions
When these risk factors are controlled for, particularly in singleton (single baby) pregnancies, the preterm birth rates for IVF pregnancies are much closer to those of natural pregnancies.
No, IVF pregnancies do not automatically require induction. Labor induction is only recommended based on standard medical reasons, just like in any other pregnancy. The decision depends on factors such as:
- Gestational age and babys readiness
- Maternal health conditions
- Fetal well-being
- Standard obstetric guidelines
Many IVF pregnancies proceed to natural labor and delivery without any need for induction.
IVF due dates are generally more accurate than natural pregnancy due dates because the exact fertilization and transfer dates are known. Unlike natural pregnancies that estimate ovulation timing, IVF provides precise dates for:
- Egg retrieval
- Fertilization
- Embryo transfer (day 3 or day 5)
This precision allows for more accurate pregnancy dating and monitoring throughout the pregnancy journey.
For frozen embryo transfer, your due date calculation depends on the embryos age at transfer :
- Day 3 embryo transfer: Add 263 days to your transfer date
- Day 5 embryo transfer: Add 261 days to your transfer date
Alternative method: Add 266 days to transfer date, then subtract the embryos age (3 or 5 days)
Your first ultrasound after IVF is typically scheduled 3-5 weeks after embryo transfer, which corresponds to 6-7 weeks of pregnancy. This timing allows doctors to:
- Detect fetal heartbeat reliably
- Determine if it is a single or multiple pregnancy
- Confirm the intrauterine pregnancy location
- Assess normal pregnancy development
The ultrasound is usually performed transvaginally for clearer, more accurate images.
The chance of twins with IVF varies but is generally higher than with natural conception :
- Natural pregnancy: About 1.2% chance of twins
- IVF pregnancy: Ranges from 6% to 25% depending on various factors
- Multiple embryo transfer: Significantly increases twin probability
- Single embryo transfer: Reduces twin risk to levels closer to natural conception
Modern practice increasingly favors single embryo transfer to minimize multiple pregnancy risks.
IVF pregnancies typically involve more intensive early monitoring :
- More frequent blood tests: Beta-hCG levels monitored every 48 hours initially
- Earlier ultrasounds: First scan at 6-7 weeks vs. 8-12 weeks for natural pregnancy
- Additional monitoring: May include more frequent check-ups in early pregnancy
- Hormone support: Often requires progesterone supplementation
After the first trimester, monitoring usually follows standard prenatal care protocols.
While IVF due dates are more precise than natural pregnancy dates, slight adjustments may occur after early ultrasounds. However, changes are typically minimal because:
- IVF provides exact fertilization timing
- Early ultrasound measurements can vary slightly
- Most adjustments are within 3-5 days
Your fertility specialist will use both transfer date calculations and ultrasound measurements to determine the most accurate due date.
PICSI యొక్క ప్రధాన ప్రయోజనం, అత్యుత్తమ వీర్యకణాన్ని ఎంచుకునే దాని అధునాతన పద్ధతి. ఈ టెక్నిక్, పరిపక్వ మరియు అపరిపక్వ వీర్యకణాల మధ్య తేడాను గుర్తించడానికి పిండ శాస్త్రవేత్తలకు (Embryologists) వీలు కల్పిస్తుంది. పరిపక్వ వీర్యకణాలు పూర్తిగా అభివృద్ధి చెంది ఉంటాయి మరియు వాటిలో DNA దెబ్బతినే లేదా క్రోమోజోముల సంఖ్యలో లోపాలు ఉండే అవకాశం తక్కువ. అందువల్ల చికిత్సలో వాడటానికి ఇవి ఉత్తమమైనవి.
PICSI, మానవ శరీరంలో సహజంగా ఉండే హైలురోనిక్ యాసిడ్ (HA) అనే పదార్థానికి వీర్యకణాలను గురిచేయడం ద్వారా పనిచేస్తుంది. ఈ ప్రక్రియలో, HAకు అతుక్కోగల సామర్థ్యం ఉన్న వీర్యకణాలను గుర్తిస్తారు, మరియు వాటినే సంతాన చికిత్సలలో ఉపయోగించడానికి ఎంపిక చేస్తారు.
సాధారణ ICSI తో PICSI ని పోలుస్తూ చేసిన పరిశోధనలలో, PICSI తో గణనీయంగా అధిక ఫలదీకరణ రేట్లు సాధించవచ్చని తేలింది. అంతేకాకుండా, దీని ద్వారా ట్రాన్స్ఫర్ చేయడానికి అనువైన పిండాల సంఖ్య పెరగడంతో పాటు, అధిక నాణ్యత గల పిండాలు ఎక్కువగా ఉత్పత్తి అవుతాయని కూడా నిరూపించబడింది.
అవును, PICSI పిండం నాణ్యతను గణనీయంగా మెరుగుపరుస్తుంది. పిండం గర్భాశయానికి అతుక్కునే (ఇంప్లాంటేషన్) సామర్థ్యం పెరగడం మరియు అన్యూప్లాయిడీస్ (క్రోమోజోముల సంఖ్యలో ఉండే అసాధారణతలు) ప్రమాదం తగ్గడమే దీనికి నిదర్శనం.
Several factors can influence the uterine environment and embryo health, including hormonal imbalances, underlying medical conditions such as PCOS or endometriosis, uterine anomalies, lifestyle factors like smoking, obesity, or stress, age, exposure to environmental toxins, and genetic or epigenetic influences.
Endometriosis can cause inflammation, scarring, and adhesions in the pelvic region, creating an unfavourable uterine environment. It can alter hormonal levels, restrict blood flow, and potentially obstruct the fallopian tubes or uterine cavity, all of which may impair embryo implantation and development.
The uterine lining, or endometrium, is essential for embryo implantation and nourishment in early pregnancy. If the lining is too thin or not receptive, it can hinder implantation and negatively affect embryo development. Maintaining a healthy, well-prepared endometrial lining is crucial for successful pregnancy.
Uterine fibroids are non-cancerous growths in the uterus that can interfere with fertility by altering the shape and function of the uterine cavity. They may block the fallopian tubes, disrupt implantation, or reduce blood flow to the embryo, ultimately impacting fertility and embryo health.
While there is no evidence that bed rest improves outcomes after embryo transfer, it’s important to relax during the “two-week wait” between the transfer and your official pregnancy test. Gentle activity is generally safe, but avoid intense physical exertion.
Yes, traveling after a blastocyst transfer is generally considered safe. There is no scientific evidence that traveling negatively impacts the success of the embryo transfer. However, it’s always a good idea to avoid stress and stay comfortable during this time.
While no specific activity guarantees implantation, maintaining a balanced and nutritious diet, getting adequate rest, avoiding stress, taking your prescribed medications (especially folic acid), and steering clear of heat exposure and harmful chemicals may help improve the chances of a successful pregnancy.
Some herbal teas contain caffeine or ingredients that may interfere with medications or fertility. It is best to avoid herbal remedies during this time unless recommended by your doctor. Always consult your fertility specialist before using any supplements or herbal products.
Yes, limiting exposure to environmental pollutants and harmful chemicals is important after a blastocyst transfer. These substances can negatively impact implantation and early pregnancy development, so aim to stay in clean, low-stress environments whenever possible.
Honestly? About 60-70% accurate within one week. If you have regular cycles, the LMP method works great. Early ultrasounds are even better. But remember, your baby has their own timeline!
Absolutely! Just use your best average or the conception date if you know it. Your first ultrasound will give you the most accurate dating anyway.
It definitely does! Most calculators (including ours) let you adjust for your actual cycle length. Every woman is different, and that’s perfectly normal.
Here’s a fun fact: only 4 out of 100 babies arrive on their exact due date! About 80% show up within two weeks either way. So think “due season” rather than “due date”.
Right after that positive pregnancy test! You’re probably dying to know anyway. Just remember your doctor might adjust it slightly after your first appointment.
While calculators don’t factor in stress, your overall health matters. Keep taking care of yourself, good nutrition, managing stress, and regular checkups all help.
Gestational age (what we usually count) starts from your last period, while fetal age starts from actual conception. That’s why pregnancies are “40 weeks” but babies develop for 38 weeks.
The calculation stays the same, but twins like to arrive fashionably early, usually around 36-37 weeks. Your Gynac Doctor will monitor you extra closely.
Yes! If your ultrasound shows a significant difference from your period-based calculation, your doctor might adjust your date. First-trimester ultrasounds are considered the most accurate.
Trust your doctor! While our calculator uses the same methods healthcare providers use, your doctor has additional information, like physical exams and ultrasound measurements.
Reputable pregnancy calculators like ours use the same formulas doctors use. The difference is that your doctor can consider your unique health factors as well.
Do not panic! Do your best to estimate, and your doctor will use an early ultrasound to date your pregnancy accurately. Some women track ovulation or conception dates instead.
If you had IVF, use your embryo transfer date! For a 3-day transfer, we add 263 days. For a 5-day transfer, we add 261 days. This is actually more accurate than traditional methods since we know the exact conception timing.
First babies especially like to take their time! Most doctors consider 37-42 weeks normal. After 41-42 weeks, your doctor will monitor you more closely and discuss induction options. Don’t stress – late babies are usually just fine!
When choosing an IVF clinic in Karimnagar, consider factors such as the clinic’s success rates, accreditation and certifications, the expertise of the medical team, available services, use of advanced technology, facility infrastructure, and overall convenience and location. Reading patient reviews and consulting with specialists can also help in making an informed decision.
Yes, the cost of IVF treatment in Karimnagar can vary from one cycle to another. Factors influencing the price include the patient’s age, medical history, type and severity of infertility, required medications, and any additional procedures such as ICSI or embryo freezing.
Yes, even if an IVF cycle is unsuccessful, there are still costs involved. These include expenses for consultations, diagnostic tests, medications, lab procedures, and embryology services. It’s important to ask the clinic for a detailed cost breakdown, including what is and isn’t refundable in case of an unsuccessful cycle.
A Class 1000 lab uses advanced filtration, strict environmental controls, and sterile protocols to prevent contamination, ensuring embryos are kept in a clean, safe environment.
Ferty9 offers advanced air filtration, high-end equipment, and expert staff to maintain superior standards of embryo care, ensuring a higher chance of success.
A class 1K cleanroom ensures high-quality embryos through precise controls, advanced equipment, and strict protocols.
A Class 1000 IVF laboratory uses high-precision incubators that regulate temperature, humidity, and air quality for optimal embryo growth.
Clean air minimizes the risk of contamination from particles, bacteria, or chemicals, protecting the embryos from potential harm.
A Class 1000 lab provides the safest, cleanest environment for embryos, enhancing their chances of healthy development and improving success rates of the latest IVF technology.
Laser-assisted hatching in IVF is a technique used to help the embryo hatch out of its protective outer shell, which is necessary for implantation into the uterus. A laser is used to create a small hole in the zona pellucida, making it easier for the embryo to hatch and implant. This technique can improve the chances of successful implantation, especially in cases where the zona pellucida is thick or hard.
Artificial intelligence in IVF helps in selecting the best embryos by analyzing their development patterns. It enhances the precision of advanced embryo selection, increasing the likelihood of a successful pregnancy.
Patients should look for clinics that adopt the latest technology in IVF treatment, including advanced systems like K System, RI Witness, and Xiltrix. Choosing centers known for their innovative approaches ensures access to high-quality care.
K-System incubators provide individual chambers for each patient and offer more stable environmental conditions compared to traditional embryo incubators. This advanced setup reduces contamination risks and supports better embryo development.
The average cost of a single IVF cycle in Vizag can range from ₹1,50,000 to ₹2,00,000, depending on factors such as the complexity of the treatment and any additional procedures required.
Yes, many fertility clinics in Vizag offer different IVF treatment packages to cater to varying needs & budgets. These packages may include consultations, diagnostic tests, medications, and the IVF procedure itself.
Most reputable fertility clinics in Vizag allow couples to customise their IVF packages based on their specific requirements. This flexibility ensures that you only pay for the services and procedures that are necessary for your treatment.
Yes, various financial assistance options are available for IVF treatments in Vizag. These include government-funded programs, insurance coverage, payment plans, discounted packages, flexible EMI options, employer assistance, and crowdfunding platforms. Exploring these options and discussing them with your fertility specialist is essential.
You will need to wait for 4 to 6 weeks following the embryo transfer and negative pregnancy test before beginning another complete IVF cycle.
The feelings of depression that women experience before IVF treatment, as well as stress from other elements of their lives, do not affect their pregnancy odds. However, a continual or chronic level of stress may disrupt your hormones, creating problems with certain aspects of IVF treatment.
Starting another IVF cycle too soon has a negative influence on the couple’s physical, mental, and emotional well-being. There may not be sufficient time for interventions or medications to have the desired effect on the successive cycle.
Preconception counseling is critical in preparing couples physically, emotionally, and mentally for the challenges of IVF, improving the chances of successful fertilization and a healthy pregnancy.
A fertility alarm, such as the Xiltrix Alarm System, enhances safety during IVF procedures by continuously monitoring critical environmental factors like temperature, humidity, and power supply. It can detect deviations from optimal conditions and trigger immediate alerts, allowing fertility clinic staff to swiftly address any issues and maintain the delicate balance required for successful embryo and gamete development.
A Fertility Alarm can provide a range of alerts to notify fertility clinic staff of potential safety concerns, including comprehensive monitoring of lab conditions, including temperature control, humidity, VOC, power failures, equipment malfunctions, and the detection of potential safety hazards like chemical spills or contamination. These alerts can be delivered through visual, audible, and digital channels, ensuring that issues are promptly identified and addressed.
Fertility alarms like the Xiltrix Alarm System can be highly customisable to accommodate the unique requirements of different fertility treatments and laboratory environments. Clinics can work with the systems manufacturer to configure the monitoring parameters, alert thresholds, and notification protocols to align with their specific needs and safety protocols.
Patients undergoing fertility treatments can benefit from using a Fertility Alarm in several ways. By enhancing the overall safety and security of the laboratory environment, the alarm system helps ensure the integrity and viability of biological samples, contributing to improved treatment outcomes. Additionally, the increased confidence in the clinic’s safety protocols can help put patients at ease and foster a greater sense of trust in the quality of care they receive.
వేడి నీటి స్నానాలు శాశ్వత సంతానలేమిని అరుదుగా కలిగిస్తాయి. కానీ, తీవ్రమైన సందర్భాల్లో, ఎక్కువ కాలం లేదా పదేపదే అధిక ఉష్ణోగ్రతలకు గురికావడం వలన దీర్ఘకాలిక నష్టం జరగవచ్చు. మీరు పిల్లల కోసం ప్లాన్ చేస్తుంటే, క్రమం తప్పకుండా వేడి నీటి స్నానాలు చేయడం మానుకోవడం మంచిది.
అవును, చల్లని నీటి స్నానాలు ఆరోగ్యకరమైన వృషణాల సంచి ఉష్ణోగ్రతను కాపాడటంలో సహాయపడటం ద్వారా సంతాన సామర్థ్యానికి మద్దతు ఇవ్వగలవు. ఎక్కువ సేపు వేడి తగలడం శుక్రకణాల నాణ్యతను దెబ్బతీస్తుంది, కానీ చల్లని ఉష్ణోగ్రతలు ఉత్తమమైన శుక్రకణాల ఉత్పత్తిని ప్రోత్సహిస్తాయి.
అవును, గర్భధారణ ప్రయత్నం తర్వాత స్నానం చేయడం సాధారణంగా సురక్షితమే, కానీ నీరు మరీ వేడిగా లేనంత వరకు మాత్రమే. అధిక ఉష్ణోగ్రతలు తాత్కాలికంగా శుక్రకణాల నాణ్యతను తగ్గించవచ్చు కాబట్టి, హాట్ టబ్లు లేదా సౌనాలకు దూరంగా ఉండండి.
ఆవిరి స్నానాలు విశ్రాంతిని ప్రోత్సహించి, రక్త ప్రసరణను మెరుగుపరుస్తాయి. అయితే, ఎక్కువ సేపు వేడికి గురికావడం వల్ల శుక్రకణాల నాణ్యత తాత్కాలికంగా ప్రభావితం కావచ్చు. మీ పునరుత్పత్తి ఆరోగ్యాన్ని కాపాడుకోవడానికి, 10-15 నిమిషాలకు పరిమితం చేసిన గోరువెచ్చని స్నానాలను ఎంచుకోండి.
Period calculator accuracy varies significantly based on individual cycle regularity. Research shows basic apps achieve only 21% accuracy for ovulation prediction, while more sophisticated methods like Ferty9’s Period Calculator can reach 70-89% accuracy for certain aspects. Next period predictions tend to be more reliable than ovulation timing for women with regular cycles, though even regular cycles naturally vary by several days.
Yes, calculators remain valuable for irregular cycles with modified expectations. Focus on pattern recognition over precise predictions, track symptoms rather than exact timing, and collect data for 6-12 months to identify subtle patterns. The educational and health awareness benefits often outweigh precise predictive accuracy for irregular cycles.
Essential information includes the first day of your last menstrual period, your average cycle length calculated from recent cycles, and typical period duration. Additional helpful data includes symptom patterns, flow intensity variations, and external factors affecting your cycles like stress or travel.
Period calculators can support conception efforts by identifying potential fertile window and helping track cycle patterns, but they have significant limitations. With only 21% accuracy for ovulation prediction, they should be combined with more reliable methods like ovulation predictor kits, basal body temperature tracking, and cervical mucus monitoring for serious conception attempts.
No. Period calculators should never be used as a contraceptive method due to low accuracy rates, inability to account for sperm survival, and unpredictable ovulation timing. Even regular cycles can shift unexpectedly due to external factors, making calculator predictions unreliable for pregnancy prevention.
Track for at least 3-4 cycles for basic pattern recognition, with optimal accuracy developing over 6-12 months. Accuracy generally improves with longer tracking history, and for irregular cycles, 12+ months may be necessary to identify patterns and triggers.
If your period is more than 7 days late, first take a pregnancy test if sexually active, then review recent lifestyle factors like stress, travel, or medication changes. Seek medical attention if the pregnancy test is negative but your period is 2+ weeks late, or if this represents a significant change from your normal pattern.
Yes, stress significantly impacts menstrual cycle regularity by disrupting reproductive hormones, delaying ovulation, and changing cycle lengths unpredictably. During stressful periods, note stress levels in your tracking, use wider prediction windows, and focus on pattern recognition rather than precise timing.
This depends on your contraceptive method. Hormonal contraceptives suppress natural cycles, making traditional calculators largely irrelevant since withdrawal bleeding isn’t a true period. Non-hormonal methods like copper IUDs allow natural cycles to continue, so calculators remain useful for pattern monitoring.
Concerning patterns include cycles consistently shorter than 21 days or longer than 35 days, complete absence of periods for 3+ months, severe pain interfering with daily activities, extremely heavy bleeding, or dramatic changes in previously established patterns. Compile 3-6 months of tracking data when consulting healthcare providers concerning changes.
Yes, perimenopause significantly reduces calculator accuracy as hormone levels fluctuate unpredictably and cycles become increasingly variable. Focus on symptom documentation rather than precise timing, track hot flashes and mood changes, and use the data to support discussions with your fertility specialist about hormone therapy options.
Several conditions significantly impact calculator effectiveness including PCOS with irregular ovulation, thyroid disorders affecting cycle regularity, endometriosis causing unpredictable symptoms, and eating disorders or extreme weight changes that can stop ovulation entirely. These conditions require specialized medical management and modified tracking approaches.
Period calculators focus on predicting menstrual timing using basic inputs like last period date and cycle length. Fertility trackers use comprehensive inputs including basal body temperature, cervical mucus, and hormone tests to identify fertile windows and confirm ovulation, making them more suitable for conception planning or natural contraception methods.
Improve accuracy through consistent daily tracking at the same time, immediate symptom recording, comprehensive documentation of physical and emotional changes, and noting lifestyle factors. Combine calculator use with additional methods like temperature tracking or cervical mucus monitoring when precision is important for health or fertility goals.
Yes, chocolate cysts can affect fertility. Healthy ovarian tissue can be invaded, damaged, and overtaken by chocolate cysts. Treatment for these cysts can be challenging, may leave ovarian scars, and may lower fertility.
No, in certain cases, chocolate cyst treatment does not require surgery. There are alternative, less intrusive therapeutic options available, and surgery is only necessary in critical cases.
No, the chocolate cyst doesn’t resolve on its own. It usually needs medical intervention, even if it is in its initial stages.
Although there is no scientific proof to support the efficacy of alternative therapies like acupuncture or dietary modifications in treating chocolate cysts, it is essential to speak with your doctor and opt for evidence-based treatment choices.
You must take birth control pills (BCPs) every day for 21 consecutive days, followed by a 7-day break during which you may take non-hormonal placebo pills or none at all. This applies to the common 28-day pill pack. The specific frequency and schedule can vary depending on the type of pill you are prescribed, so always follow the guidance of your healthcare provider.
No, birth control pills only prevent pregnancy and do not provide any protection against sexually transmitted infections (STIs). To reduce your risk of STIs, it is important to use barrier protection methods such as condoms during sexual activity.
Birth control pills can be used from the age of 20 and are generally effective until menopause. However, they may not be recommended for everyone. For example, if you are over 35 and smoke, your doctor may advise against using BCPs due to an increased risk of cardiovascular complications. Always consult your healthcare provider to determine if BCPs are appropriate for your individual health profile.
If you miss one active pill, take it as soon as you remember, even if that means taking two pills in one day. Then continue with your regular schedule. If you miss a pill by more than 12 hours, use a backup method like condoms for the next seven days. Its important to follow the instructions provided with your specific pill pack and consult your doctor if you are unsure.
Laparoscopy and hysteroscopy are surgical procedures that cause minimal tissue damage but differ in focus and approach. Laparoscopy examines and treats issues in the abdominal and pelvic regions, such as endometriosis or uterine fibroids. On the other hand, hysteroscopy examines and treats conditions within the uterine cavity, such as polyps or adhesions.
The duration of fertility surgery can vary and depends on the specific procedure and the case’s complexity. Generally, a hysteroscopy or laparoscopy can take anywhere from 30 minutes to 2 hours, while more complex procedures, such as a myomectomy, may take 2 to 4 hours.
As with any other surgical procedure, there are some risks associated with fertility surgery, which may include infection, bleeding at the incision site, damage to surrounding organs, or reactions to anaesthesia. However, these risks are generally low, and fertility surgeries are considered safe and effective when performed by experienced gynaecological surgeons.
The timeline for trying to conceive after fertility surgery can vary and depends on the specific procedure and the individuals recovery. In general, gynaecological surgeons may advise patients to wait 1-3 menstrual cycles before attempting to conceive to allow for proper healing and recovery.
Additional costs in IVF treatments can include genetic testing of embryos, assisted hatching, embryo freezing and storage, and any necessary surgical procedures. It’s essential to request a complete cost breakdown from your clinic to avoid surprises and make informed decisions.
Yes, more affordable alternatives like intrauterine insemination (IUI) or ovulation induction may be suitable depending on your fertility condition. These treatments typically involve fewer procedures and lower medication costs, making them budget-friendly options to explore with your fertility specialist.
To find a cost-effective IVF clinic in Vijayawada, evaluate clinics based on transparent pricing, success rates, available technologies, and patient reviews. Scheduling consultations with multiple clinics can help you compare costs and services to make the best decision for your fertility journey.
You can estimate the total cost of your IVF cycle by requesting a detailed quote from your clinic. This should cover all stages of treatment—initial consultations, medications, lab work, embryo transfer, follow-ups, and any additional services. A transparent estimate ensures financial clarity throughout your treatment process.
అవును, ఇది పూర్తిగా సాధారణం. చాలా మంది ఆరోగ్యకరమైన జంటలు చురుకుగా ప్రయత్నించిన 6-12 నెలల్లో గర్భం దాలుస్తారు. మీరు 35 సంవత్సరాల కంటే తక్కువ వయస్సు కలిగి ఉంటే, వైద్యులు సాధారణంగా సహాయం కోసం వెళ్ళే ముందు ఒక సంవత్సరం వరకు ప్రయత్నించమని సలహా ఇస్తారు.
మీరు 35 సంవత్సరాల కంటే తక్కువ వయస్సు కలిగి ఉండి, 12 నెలలు ప్రయత్నించినా గర్భం దాల్చకపోతే లేదా మీరు 35 సంవత్సరాల కంటే ఎక్కువ వయస్సు కలిగి ఉండి, 6 నెలలు ప్రయత్నించినా గర్భం దాల్చకపోతే సంతానోత్పత్తి నిపుణుడిని కలవడాన్ని పరిగణించండి. వయస్సు సంతానోత్పత్తిని గణనీయంగా ప్రభావితం చేస్తుంది కాబట్టి, ఎక్కువ వయస్సు ఉన్నవారు త్వరగా సహాయం తీసుకోవడం మంచిది.
అవును, ఒత్తిడి మీ గర్భం దాల్చే సామర్థ్యంలో ముఖ్యమైన పాత్ర పోషిస్తుంది. అధిక ఒత్తిడి స్థాయిలు మహిళల్లో ఒవ్యులేషన్ను నియంత్రించే మరియు పురుషుల్లో వీర్యం ఉత్పత్తిని నియంత్రించే హార్మోన్ల యొక్క సున్నితమైన సమతుల్యతను దెబ్బతీస్తాయి. ఇది లిబిడోను కూడా ప్రభావితం చేస్తుంది, దీని వలన క్రమం తప్పకుండా లైంగిక సంపర్కం తక్కువగా ఉంటుంది. గర్భం దాల్చడానికి ప్రయత్నిస్తున్నప్పుడు విశ్రాంతి పద్ధతులు, వ్యాయామం లేదా కౌన్సెలింగ్ ద్వారా ఒత్తిడిని నిర్వహించడం ప్రయోజనకరంగా ఉంటుంది.
మీరు మీ ఒవ్యులేషన్ను ట్రాక్ చేయడానికి మరియు మీ ఫలవంతమైన సమయాన్ని గుర్తించడానికి అనేక మార్గాలు ఉన్నాయి:
బేసల్ బాడీ టెంపరేచర్: ప్రతి ఉదయం విశ్రాంతిగా ఉన్నప్పుడు మీ ఉష్ణోగ్రతను ట్రాక్ చేయడం ఒవ్యులేషన్ జరిగిన తర్వాత స్వల్ప పెరుగుదలను వెల్లడిస్తుంది.
ఒవ్యులేషన్ ప్రిడిక్టర్ కిట్లు (OPKs): ఈ ఓవర్-ది-కౌంటర్ కిట్లు మీ మూత్రంలో లూటినైజింగ్ హార్మోన్ (LH) పెరుగుదలను గుర్తించాయి, ఇది సాధారణంగా ఒవ్యులేషన్కు 24-48 గంటల ముందు జరుగుతుంది.
గర్భాశయ శ్లేష్మం యొక్క మార్పులను గమనించడం: మీ ఋతు చక్రం అంతటా గర్భాశయ శ్లేష్మం స్థిరత్వం మరియు రూపాన్ని గమనించడం ద్వారా మీరు ఎప్పుడు అత్యంత ఫలవంతంగా ఉన్నారో తెలుసుకోవచ్చు.
ఆరోగ్యకరమైన జీవనశైలిని అవలంబించడం మీ గర్భం దాల్చే అవకాశాలను గణనీయంగా మెరుగుపరుస్తుంది. పరిగణించవలసిన ముఖ్యమైన మార్పులు:
సమతుల్య ఆహారం: పండ్లు, కూరగాయలు, తృణధాన్యాలు మరియు తక్కువ కొవ్వు కలిగిన ప్రోటీన్లతో నిండిన పోషక ఆహారాన్ని తీసుకోండి. ఫోలేట్, జింక్, విటమిన్ డి వంటి అవసరమైన పోషకాలను పొందండి.
క్రమం తప్పకుండా వ్యాయామం: మితమైన వ్యాయామం ఆరోగ్యకరమైన బరువును నిర్వహించడంలో మరియు పునరుత్పత్తి సామర్థ్యాన్ని మెరుగుపరచడంలో సహాయపడుతుంది.
ఆరోగ్యకరమైన బరువు: BMI ని 18.5-24.9 మధ్య నిర్వహించడం హార్మోన్ల సమతుల్యతకు అవసరం.
ఆల్కహాల్ మరియు కెఫైన్ పరిమితి: మితంగా తీసుకోవడం మంచిది, అధికంగా తీసుకోవడం సంతానోత్పత్తిని దెబ్బతీస్తుంది.
ఒత్తిడి నిర్వహణ: యోగా, ధ్యానం, లేదా అభిరుచులపై సమయం కేటాయించడం ఒత్తిడి తగ్గించడంలో సహాయపడుతుంది.
Coughing does not affect embryo implantation or compromise IVF success. The embryo is securely cushioned inside the uterus and has already burrowed into the uterine lining. Multiple fertility experts confirm that coughing is a natural physiological response that poses no risk to implantation. However, if a persistent cough causes discomfort, patients should consult their doctor.
Sneezing has no impact on embryo implantation. The embryo is safely positioned within the uterine cavity and cannot be dislodged by normal bodily functions. Medical research shows that the embryo is protected by the natural cushioning of the uterine environment, allowing patients to sneeze without worrying about affecting their treatment outcome.
During the crucial waiting period, patients should avoid:
- Exposure to excessive heat sources
- Areas with heavy pollution or chemical fumes
- High-traffic areas during peak times
- Stressful environments or situations
- Taking home pregnancy tests before the scheduled blood test
Folic acid supplementation is vital for pregnancy. Research indicates that proper folic acid supplementation can enhance IVF success rates and reduce the risk of neural tube defects. The Centres for Disease Control & Prevention recommends specific daily intake levels:
| Category | Recommended Daily Intake |
|---|---|
| Standard Dose | 400-800 micrograms |
| High-risk Cases | Additional dosage as prescribed |
దగ్గు పిండం ఇంప్లాంటేషన్ను ప్రభావితం చేయదు లేదా IVF విజయాన్ని దెబ్బతీయదు. పిండం గర్భాశయంలో సురక్షితంగా ఉంటుంది మరియు ఇప్పటికే గర్భాశయ పొరలోకి చొచ్చుకుపోయింది. చాలా మంది సంతానోత్పత్తి నిపుణులు దగ్గు అనేది సహజమైన శారీరక ప్రతిస్పందన అని మరియు అది ఇంప్లాంటేషన్కు ఎటువంటి ప్రమాదం కలిగించదని ధృవీకరిస్తున్నారు. అయితే, నిరంతర దగ్గు అసౌకర్యాన్ని కలిగిస్తే, రోగులు వారి వైద్యుడిని సంప్రదించాలి.
తుమ్మడం పిండం ఇంప్లాంటేషన్పై ఎటువంటి ప్రభావం చూపదు. పిండం గర్భాశయ కుహరంలో సురక్షితంగా ఉంచబడుతుంది మరియు సాధారణ శారీరక విధుల ద్వారా కదలదు. వైద్య పరిశోధన ప్రకారం, పిండం గర్భాశయ వాతావరణం యొక్క సహజమైన రక్షణ ద్వారా రక్షించబడుతుంది, ఇది చికిత్స ఫలితాన్ని ప్రభావితం చేస్తుందనే భయం లేకుండా రోగులు తుమ్మడానికి అనుమతిస్తుంది.
కీలకమైన నిరీక్షణ కాలంలో, రోగులు వీటిని నివారించాలి:
• అధిక వేడికి గురికావడం
• ఎక్కువ కాలుష్యం లేదా రసాయన పొగలు ఉన్న ప్రాంతాలు
• రద్దీ సమయాల్లో ఎక్కువ ట్రాఫిక్ ఉన్న ప్రాంతాలు
• ఒత్తిడితో కూడిన వాతావరణాలు లేదా పరిస్థితులు
• షెడ్యూల్ చేసిన రక్త పరీక్షకు ముందు ఇంటి వద్ద ప్రెగ్నెన్సీ పరీక్షలు చేయడం
ఫోలిక్ యాసిడ్ సప్లిమెంటేషన్ గర్భధారణకు చాలా అవసరం. సరైన ఫోలిక్ యాసిడ్ సప్లిమెంటేషన్ IVF విజయాల రేటును పెంచుతుందని మరియు నాడీ నాళ లోపాల ప్రమాదాన్ని తగ్గిస్తుందని పరిశోధన సూచిస్తుంది.
సెంటర్స్ ఫర్ డిసీజ్ కంట్రోల్ & ప్రివెన్షన్ నిర్దిష్ట రోజువారీ తీసుకోవలసిన స్థాయిలను సిఫార్సు చేస్తుంది:
వర్గం | సిఫార్సు చేసిన రోజువారీ తీసుకోవలసిన మోతాదు
సాధారణ సందర్భాలు: 400-800 మైక్రోగ్రాములు
అధిక-ప్రమాద కేసులు: సూచించిన విధంగా అదనపు మోతాదు
అవును, PCOD నెలసరి క్రమం తప్పడానికి ప్రధాన కారణం. ఇది ఒక హార్మోన్ల సమస్య, దీనిలో అండాశయాలు ఎక్కువగా ఆండ్రోజెన్లను (పురుష హార్మోన్లు) ఉత్పత్తి చేస్తాయి. పెరిగిన పురుష హార్మోన్ స్థాయిలు అండం పరిపక్వత మరియు విడుదలను దెబ్బతీస్తాయి, దీనివల్ల నెలసరి క్రమం తప్పుతుంది. అండాశయాలలో అపరిపక్వ గుడ్లతో కూడిన తిత్తులు ఏర్పడవచ్చు, ఇది కూడా నెలసరి క్రమం తప్పడానికి కారణమవుతుంది.
PCOD యొక్క లక్షణాలు వివిధ రకాలుగా ప్రభావం చూపవచ్చు, అవి: నెలసరి క్రమం తప్పకుండా రావడం, నెలసరి పూర్తిగా ఆగిపోవడం, ఎక్కువ రక్తస్రావం కావడం మరియు నొప్పిగా ఉండటం. తక్కువ తరచుగా వచ్చే నెలసరి అంటే సంవత్సరానికి ఎనిమిది కంటే తక్కువ సార్లు రావడం, మరియు చక్రం 35 రోజుల కంటే ఎక్కువ ఉండటం. నెలసరి మూడు నెలలు లేదా అంతకంటే ఎక్కువ కాలం రాకపోవడం, మరియు క్రమం తప్పని నెలసరి వచ్చే సమయం చాలా మారుతూ ఉండటం వల్ల తర్వాతి నెలసరి ఎప్పుడు వస్తుందో ఊహించడం కష్టం అవుతుంది.
PCOD ఉన్న మహిళలకు సరైన బరువును నిర్వహణ చేయడం చాలా ముఖ్యం, ఎందుకంటే ఇన్సులిన్ నిరోధకత పెరిగిన ఆండ్రోజెన్ ఉత్పత్తికి దారితీస్తుంది, ఇది నెలసరిలో క్రమరాహిత్యాలకు కారణమవుతుంది. అధిక బరువు, ముఖ్యంగా పొత్తికడుపులో కొవ్వు, ఈ సమస్యలను మరింత తీవ్రతరం చేస్తుంది. బరువు తగ్గడం ఇన్సులిన్ సున్నితత్వాన్ని మెరుగుపరుస్తుంది మరియు ఆండ్రోజెన్ స్థాయిని తగ్గిస్తుంది, ఫలితంగా PCOD సంబంధిత సమస్యలు కూడా తగ్గుతాయి.
నెలసరి క్రమం తప్పకుండా రావడానికి (PCOD) సహజ నివారణలలో తక్కువ గ్లైసెమిక్ ఇండెక్స్ (GI) ఆహారం, యాంటీ ఇన్ఫ్లమేటరి ఆహారం, ప్రాసెస్ చేసిన ఆహారాలను తగ్గించడం, మూలికా సప్లిమెంట్లు, ఒత్తిడి నిర్వహణ పద్ధతులు మరియు తగినంత నిద్ర ఉన్నాయి. ఈ నివారణలు మొత్తం ఆరోగ్యాన్ని మెరుగుపరుస్తాయి మరియు PCOD లక్షణాలను తగ్గిస్తాయి, అయితే వీటి గురించి ఆరోగ్య సంరక్షణ నిపుణుడితో చర్చించాలి. క్రమం తప్పకుండా వ్యాయామం చేయడం ఇన్సులిన్ సున్నితత్వాన్ని, బరువు నిర్వహణను మరియు హార్మోన్ల సమతుల్యతను మెరుగుపరుస్తుంది.
మహిళా భాగస్వాములలో నెలసరి చక్రాన్ని క్రమబద్ధీకరించడానికి వైద్య చికిత్సలలో హార్మోన్ల నియంత్రణ, ప్రొజెస్టిన్ థెరపీ, డయాబెటిస్ నియంత్రణ మందులు, యాంటీ-ఆండ్రోజెన్ మందులు మరియు ఒవ్యులేషన్ ను ప్రోత్సహించే మందులు ఉండవచ్చు. సంతానోత్పత్తి నిపుణులు సూచించిన తగిన చికిత్సలు లక్షణాలను నిర్వహించడానికి, దీర్ఘకాలిక సమస్యలను తగ్గించడానికి మరియు సాధారణ ఒవ్యులేషన్ ను ప్రోత్సహించడానికి మరియు గర్భం దాల్చే అవకాశాలను మెరుగుపరచడానికి సహాయపడతాయి.
PCODని నిర్వహించడానికి మరియు నెలసరి క్రమబద్ధతను మెరుగుపరచడానికి జీవనశైలి మార్పులు చాలా కీలకం. ప్రాసెస్ చేసిన మరియు శుద్ధి చేసిన చక్కెరలు తక్కువగా ఉండే, ఫైబర్ మరియు తృణధాన్యాలు ఎక్కువగా ఉండే సమతుల్య ఆహారం రక్తంలో చక్కెర స్థాయిలను స్థిరీకరిస్తుంది, ఇన్సులిన్ సున్నితత్వాన్ని మెరుగుపరుస్తుంది మరియు హార్మోన్ల సమతుల్యతకు మద్దతు ఇస్తుంది. క్రమం తప్పకుండా వ్యాయామం చేయడం, బరువు నిర్వహణ మరియు ఆరోగ్యకరమైన బరువును నిర్వహించుకోవటం ముఖ్యమైన అంశాలు. ఒత్తిడి నిర్వహణ మరియు తగినంత నిద్ర హార్మోన్ల ఆరోగ్యానికి, కార్టిసాల్ స్థాయిలను నియంత్రించడానికి మరియు నెలసరి చక్రంలో పాల్గొనే ఇతర హార్మోన్లను ప్రభావితం చేయడానికి చాలా అవసరం.
PCOS మరియు క్రమం తప్పని నెలసరితో గర్భం దాల్చడం ఒవ్యులేషన్ చక్రాలు దెబ్బతినడం వల్ల కష్టంగా ఉంటుంది. క్రమం తప్పని నెలసరిని నివారించడానికి, ఆరోగ్యకరమైన ఆహారం, క్రమం తప్పకుండా వ్యాయామం మరియు ఒత్తిడిని తగ్గించడం వంటి ఆరోగ్యకరమైన జీవనశైలిని అనుసరించండి. జీవనశైలి మార్పులు సరిపోకపోతే, సంతానోత్పత్తి నిపుణులు సూచించిన వైద్య సహాయం, సమయం చూసుకొని కలవడం, ఇంట్రాట్యూటరైన్ ఇన్సెమినేషన్ (IUI) మరియు ఇన్ విట్రో ఫెర్టిలైజేషన్ (IVF) ఉపయోగించవచ్చు. ఈ చికిత్సలు ఒవ్యులేషన్ మరియు ఫలదీకరణ సవాళ్లను అధిగమించడానికి సహాయపడతాయి.
Freezing your eggs offers several benefits, including:
- Preserving Fertility: By freezing eggs at a younger age, women can effectively pause their biological clock and increase the likelihood of conceiving later in life.
- Flexibility and Control: Egg freezing gives women the freedom to focus on personal or professional goals without compromising their future fertility.
- Overcoming Age-Related Fertility Decline: Freezing eggs earlier in life helps bypass natural fertility decline associated with aging.
- Medical Reasons: In some cases, egg freezing is advised before medical treatments, such as chemotherapy, that could negatively impact fertility.
The success rate of egg freezing varies based on several factors, including the woman’s age at the time of freezing, the number and quality of eggs retrieved, and the clinic’s procedures. Generally, freezing eggs at a younger age improves the chances of successful fertilisation and pregnancy later.
Frozen eggs can be stored for several years—often a decade or longer—depending on the clinic’s policies and storage conditions. With modern cryopreservation techniques, the long-term storage of eggs is considered safe and effective, although prolonged storage may slightly impact viability.
The cost of egg freezing can vary widely depending on factors such as the location, fertility clinic, and treatment plan. Expenses typically include initial consultations, hormone treatments, egg retrieval, and ongoing storage fees. It is advisable to consult directly with fertility clinics for detailed pricing.
Yes, frozen eggs can be used for in vitro fertilisation (IVF) when a woman decides to conceive. The process involves thawing the eggs, fertilising them with sperm in a lab, and then transferring the resulting embryos to the uterus to achieve pregnancy.
గుడ్లు భద్రపరచడం వల్ల చాలా లాభాలు ఉన్నాయి, వాటిలో కొన్ని ఇక్కడ ఉన్నాయి:
సంతానోత్పత్తిని కాపాడుతుంది: చిన్న వయస్సులో గుడ్లను భద్రపరచడం ద్వారా, మహిళలు తమ జీవ గడియారాన్ని ఆపగలరు మరియు తరువాత జీవితంలో గర్భం దాల్చే అవకాశాలను పెంచుకోగలరు.
ఫ్లెక్సిబిలిటీ మరియు నియంత్రణ: గుడ్లు భద్రపరచడం వలన మహిళలు తమ భవిష్యత్తు సంతానోత్పత్తిని రాజీ పడకుండా వ్యక్తిగత లేదా వృత్తిపరమైన లక్ష్యాలపై దృష్టి పెట్టడానికి స్వేచ్ఛ లభిస్తుంది.
వయస్సు సంబంధిత సంతానోత్పత్తి క్షీణతను అధిగమించడం: చిన్న వయస్సులో గుడ్లను భద్రపరచడం వృద్ధాప్యం వల్ల వచ్చే సహజమైన సంతానోత్పత్తి క్షీణతను దాటవేయడానికి సహాయపడుతుంది.
వైద్య కారణాలు: కొన్ని సందర్భాల్లో, కీమోథెరపీ వంటి సంతానోత్పత్తిపై ప్రతికూల ప్రభావం చూపే వైద్య చికిత్సలకు ముందు గుడ్లు భద్రపరచమని సలహా ఇస్తారు.
గుడ్లు భద్రపరచడంలో విజయం రేటు అనేక అంశాలపై ఆధారపడి మారుతుంది, వాటిలో గుడ్లు భద్రపరిచే సమయంలో మహిళ వయస్సు, సేకరించిన గుడ్ల సంఖ్య మరియు నాణ్యత మరియు క్లినిక్ యొక్క విధానాలు ఉన్నాయి. సాధారణంగా, చిన్న వయస్సులో గుడ్లను భద్రపరచడం తరువాత విజయవంతమైన ఫలదీకరణం మరియు గర్భం దాల్చే అవకాశాలను మెరుగుపరుస్తుంది.
గుడ్లను భద్రపరిచే సమయం క్లినిక్ విధానాలు మరియు నిల్వ పరిస్థితులపై ఆధారపడి చాలా సంవత్సరాలు ఉండవచ్చు. తరచుగా పదేళ్లు లేదా అంతకంటే ఎక్కువ. ఆధునిక క్రయోప్రిజర్వేషన్ పద్ధతులతో, గుడ్ల యొక్క దీర్ఘకాలిక నిల్వ సురక్షితమైనది మరియు ప్రభావవంతమైనదిగా పరిగణించబడుతుంది, అయినప్పటికీ ఎక్కువ కాలం నిల్వ ఉంచడం వలన వాటి నాణ్యత కొద్దిగా తగ్గవచ్చు.
గుడ్లు భద్రపరచడానికి ఎంత ఖర్చు అవుతుంది అనేది ప్రాంతం, ఫెర్టిలిటీ క్లినిక్ మరియు చికిత్స ప్రణాళిక వంటి అంశాలపై విస్తృతంగా మారుతూ ఉంటుంది. ఖర్చులలో సాధారణంగా మొదటిసారి సంప్రదింపులు, హార్మోన్ల చికిత్సలు, గుడ్లు తీయడం మరియు కొనసాగుతున్న నిల్వ రుసుములు ఉంటాయి. వివరణాత్మక ధరల కోసం నేరుగా ఫెర్టిలిటీ క్లినిక్లను సంప్రదించడం మంచిది.
అవును, ఒక మహిళ గర్భం దాల్చాలని నిర్ణయించుకున్నప్పుడు, భద్రపరిచిన గుడ్లను ఇన్ విట్రో ఫెర్టిలైజేషన్ (IVF) కోసం ఉపయోగించవచ్చు. ఈ ప్రక్రియలో గుడ్లను కరిగించడం, ల్యాబ్లో స్పెర్మ్తో ఫలదీకరణం చేయడం మరియు తరువాత వచ్చిన పిండాలను గర్భాశయానికి బదిలీ చేయడం ద్వారా గర్భం దాల్చడం జరుగుతుంది.
శతాబ్దాలుగా, ఆయుర్వేద వైద్యులు అశ్వగంధ, శతావరి మరియు గుడుచి వంటి మూలికలను హార్మోన్లను నియంత్రించడానికి ఉపయోగిస్తున్నారు. ఆయుర్వేద మూలికల సహాయంతో ఒత్తిడి మరియు ఆందోళనను తగ్గించవచ్చు. అధిక స్థాయిలో ఒత్తిడి శరీరంలో ఎక్కువ కార్టిసాల్ ఉత్పత్తికి కారణమవుతుంది, ఇది స్త్రీలలో హార్మోన్ సంశ్లేషణ మరియు నియంత్రణకు ఆటంకం కలిగిస్తుంది. కాబట్టి, ఆయుర్వేద మూలికలు పరోక్షంగా హార్మోన్ల అసాధారణ స్థాయిలను సరిచేయడానికి సహాయపడతాయి.
ఖచ్చితంగా, ఆయుర్వేద మూలికలను ఆధునిక సంతానోత్పత్తి చికిత్సలతో ఉపయోగించవచ్చు. ఈ మూలికలు పురుషులు మరియు స్త్రీలలో వంధ్యత్వ సమస్యలను అధిగమించడానికి సహాయపడతాయి. ఆధునిక మందులతో ఆయుర్వేద మూలికా నివారణలను కలపడం రోగులకు ఎక్కువ చికిత్స ఎంపికలను అందించవచ్చు, దుష్ప్రభావాలను తగ్గించవచ్చు మరియు మొత్తం చికిత్సా ఫలితాలను మెరుగుపరచవచ్చు.
కొన్ని మూలికా మిశ్రమాలలో సీసం మరియు పాదరసం వంటి ప్రమాదకరమైన భారీ లోహాలు ఉండవచ్చు, ఇవి ఇన్-ఫెర్టిలిటీ వంటి తీవ్రమైన ఆరోగ్య సమస్యలకు కారణమవుతాయి. కొన్ని మూలికలు కడుపు నొప్పి, అసౌకర్యం, విరేచనాలు, వికారం, ఎక్కిళ్ళు, వాంతులు, త్రేనుపులు, తలనొప్పి, కడుపులో అసౌకర్యం మరియు వదులుగా ఉండే మలానికి కారణం కావచ్చు.
తల్లి మరియు తండ్రి యొక్క పునరుత్పత్తి ఆరోగ్యాన్ని మెరుగుపరచడానికి మరియు వీర్యం లేదా అండం మరియు గర్భాశయం సరైన స్థితిలో ఉన్నాయని నిర్ధారించడానికి, ఆయుర్వేద మూలికలతో ఫలితాలు చూడటానికి కనీసం మూడు నెలలు మరియు ఆదర్శంగా పన్నెండు నెలల సమయం పడుతుందని భావిస్తారు.
When choosing an IVF clinic in Rajahmundry, consider factors like the clinic’s success rates, accreditation, the experience and expertise of the medical professionals, the range of fertility services offered, the availability of advanced technology, and the overall patient experience. Location and ease of access also matter for convenience during treatment. Researching patient reviews and consulting directly with the clinic can help you make an informed decision.
Yes, the cost of IVF treatment in Rajahmundry can vary across different cycles. Factors such as the individual’s fertility condition, response to medication, additional procedures required, and the length of treatment can impact the overall cost. Some patients may need more than one cycle, which increases expenses accordingly.
Yes, there are costs involved even in unsuccessful IVF cycles in Rajahmundry. Clinics generally charge for each individual cycle, regardless of its outcome. This includes fees for consultations, medications, diagnostic tests, procedures, and laboratory services. Patients should be informed about all potential costs and financial policies beforehand.
IVF payments in Rajahmundry are typically structured in phases. An initial payment is usually required at the beginning of the treatment. The remaining amount is often paid in stages, with the final payment due around the time of the egg retrieval procedure. The exact payment schedule may vary based on the clinic’s specific package and policies.
తప్పుడు పాజిటివ్ ఫలితాలకు అత్యంత సాధారణ కారణాలలో కెమికల్ ప్రెగ్నెన్సీలు, కొన్ని మందుల ప్రభావాలు, మరియు పరీక్ష చేయడంలో తప్పులు ఉన్నాయి. డాక్టర్లు ఈ క్రింది వాటిని ప్రాథమిక కారకాలుగా గుర్తిస్తారు: ఇటీవలి సంతాన సాఫల్య చికిత్సలు, hCG కలిగిన కొన్ని మందులు వాడటం, గర్భ నష్టం జరిగిన వెంటనే పరీక్షించడం, సూచించిన సమయం దాటిన తర్వాత ఫలితాలను చదవడం, మరియు గడువు ముగిసిన టెస్ట్ కిట్లను ఉపయోగించడం.
అవును. టెస్టులో నెగటివ్ ఫలితం వచ్చినప్పటికీ, నెలసరి ఆలస్యం అయితే మీరు గర్భవతి అయ్యే అవకాశం ఉంది. ప్రత్యేకించి మీరు చాలా ముందుగా టెస్ట్ చేసి ఉంటే, గుర్తించడానికి మీ శరీరంలో హార్మోన్ల స్థాయిలు చాలా తక్కువగా ఉండవచ్చు. గర్భం అని అనుమానం ఉంటే 48–72 గంటలు వేచి ఉండి, మళ్ళీ పరీక్షించుకోవాలని డాక్టర్లు సిఫార్సు చేస్తారు.
చాలా ప్రెగ్నెన్సీ టెస్టులు నెలసరి తప్పిపోయిన మరుసటి రోజు చేసుకుంటే అత్యంత కచ్చితమైన ఫలితాలను ఇస్తాయి. అయితే, కొన్ని అత్యంత సున్నితమైన (highly sensitive) టెస్టులు గర్భధారణ హార్మోన్లను ముందుగానే గుర్తించగలవు. అత్యుత్తమ కచ్చితత్వం కోసం, హార్మోన్ల గాఢత ఎక్కువగా ఉండే ఉదయం పూట పరీక్ష జరగాలి.
రాజమండ్రిలో ఒక IVF క్లినిక్ను ఎంచుకునేటప్పుడు, క్లినిక్ యొక్క సక్సెస్ రేట్లు (విజయ శాతాలు), అక్రిడిటేషన్ (గుర్తింపు), వైద్య నిపుణుల అనుభవం మరియు నైపుణ్యం, అందించే ఫెర్టిలిటీ సేవల పరిధి, అధునాతన సాంకేతిక పరిజ్ఞానం లభ్యత, మరియు మొత్తం రోగి అనుభవం వంటి అంశాలను పరిగణించండి. చికిత్స సమయంలో సౌలభ్యం కోసం క్లినిక్ ఉన్న ప్రదేశం మరియు సులభంగా చేరుకోగలిగే సౌకర్యం కూడా ముఖ్యమైనవి. రోగి సమీక్షలను పరిశోధించడం మరియు క్లినిక్తో నేరుగా సంప్రదించడం మీరు సమాచారంతో కూడిన నిర్ణయం తీసుకోవడంలో సహాయపడుతుంది.
అవును, రాజమండ్రిలో IVF చికిత్స ఖర్చు వివిధ సైకిళ్లలో మారవచ్చు. వ్యక్తి యొక్క సంతానోత్పత్తి పరిస్థితి, మందులకు స్పందన, అవసరమైన అదనపు ప్రక్రియలు, మరియు చికిత్స వ్యవధి వంటి అంశాలు మొత్తం ఖర్చును ప్రభావితం చేస్తాయి. కొంతమంది రోగులకు ఒకటి కంటే ఎక్కువ సైకిళ్లు అవసరం కావచ్చు, ఇది తదనుగుణంగా ఖర్చులను పెంచుతుంది.
అవును, రాజమండ్రిలో విఫలమైన IVF సైకిళ్లలో కూడా ఖర్చులు ఉంటాయి. క్లినిక్లు సాధారణంగా ప్రతి ఒక్క సైకిల్కు, దాని ఫలితంతో సంబంధం లేకుండా ఛార్జ్ చేస్తాయి. ఇందులో కన్సల్టేషన్లు, మందులు, రోగనిర్ధారణ పరీక్షలు, ప్రక్రియలు, మరియు ప్రయోగశాల సేవల కోసం రుసుములు ఉంటాయి. రోగులకు ముందుగానే అన్ని సంభావ్య ఖర్చులు మరియు ఆర్థిక విధానాల గురించి తెలియజేయాలి.
రాజమండ్రిలో IVF చెల్లింపులు సాధారణంగా దశలవారీగా నిర్మించబడతాయి. చికిత్స ప్రారంభంలో సాధారణంగా ఒక ప్రారంభ చెల్లింపు అవసరం అవుతుంది. మిగిలిన మొత్తం తరచుగా దశలవారీగా చెల్లించబడుతుంది, చివరి చెల్లింపు అండాల సేకరణ (egg retrieval) ప్రక్రియ సమయంలో చెల్లించాల్సి ఉంటుంది. ఖచ్చితమైన చెల్లింపు షెడ్యూల్ క్లినిక్ యొక్క నిర్దిష్ట ప్యాకేజీ మరియు విధానాలపై ఆధారపడి మారవచ్చు.
Low libido in females can result from various factors such as hormonal imbalances, stress, fatigue, certain medications, and underlying medical conditions. Psychological factors, relationship issues, or lifestyle habits, such as excessive masturbation, can also contribute to a reduced sex drive. Identifying the root cause is crucial for effective treatment.
Preventing female sexual dysfunction involves a holistic approach that includes maintaining a healthy lifestyle, managing stress levels, avoiding excessive masturbation, staying physically active, and seeking early treatment for underlying medical or psychological issues. Open communication with a partner and regular gynecological checkups are also essential.
There are numerous myths and misconceptions surrounding masturbation. Some common but false beliefs include:
- Masturbation can cause blindness.
- Masturbation can lead to hair loss.
- Masturbation can cause mental illness.
- Masturbation can result in infertility.
- Masturbation is immoral or sinful.
While these myths are not scientifically supported, over-masturbation may lead to concerns such as neural fatigue or physical exhaustion if practiced excessively. Moderation and awareness are key.
అవును, అనేక వైద్య పరిస్థితులు ప్రెగ్నెన్సీ టెస్ట్ ఫలితాలను ప్రభావితం చేస్తాయి. వీటిలో కొన్ని రకాల అండాశయ తిత్తులు (ovarian cysts), పిట్యూటరీ గ్రంథి లోపాలు, మరియు కొన్ని అరుదైన కణితులు ఉన్నాయి. ఈ పరిస్థితులు ఉన్న వ్యక్తులు సరైన మూల్యాంకనం మరియు పరీక్ష మార్గదర్శకత్వం కోసం డాక్టర్లను సంప్రదించాలి.
గరిష్ట కచ్చితత్వం కోసం నెలసరి తప్పిపోయిన 1-2 రోజుల తర్వాత పరీక్ష జరగాలి. గాఢమైన హార్మోన్ల స్థాయిల కారణంగా ఉదయం పూట చేసే పరీక్ష అత్యంత నమ్మదగిన ఫలితాలను అందిస్తుంది. ఒకవేళ అనిశ్చితి ఉంటే, నిర్ధారితమైన ఫలితాల కోసం నెలసరి తప్పిపోయిన ఒక వారం తర్వాత వేచి ఉండాలని డాక్టర్లు సిఫార్సు చేస్తారు.
Masturbation is a physically strenuous and energy-intensive activity. During the act, the body expends energy, which may result in feelings of tiredness or fatigue afterward. While occasional masturbation typically does not cause significant energy depletion, excessive frequency might leave you feeling more exhausted than anticipated.
Masturbation itself does not cause erectile dysfunction (ED). This is a common misconception. In fact, masturbation is a normal and healthy sexual activity. However, psychological factors such as guilt, stress, or anxiety related to excessive masturbation may indirectly impact sexual performance. If concerns persist, consulting a healthcare professional is advisable.
Masturbation does not negatively impact sperm count or fertility. While each ejaculation temporarily reduces sperm levels, the body quickly replenishes sperm. Masturbating, even frequently, does not have any long-term adverse effects on fertility or overall sperm health.
Masturbation can influence sleep in different ways. For many, it induces relaxation and helps in falling asleep more easily. However, if done excessively or compulsively, it may interfere with regular sleep patterns and overall sleep quality. Maintaining moderation is key to supporting a healthy sleep cycle.
In some cases, masturbation can become compulsive and mimic addiction-like behaviors. This may interfere with daily responsibilities or relationships. If you feel that masturbation is impacting your life negatively, its important to seek help from a healthcare professional. There no shame in asking for support—your doctor may recommend therapy to help manage the behavior and address any underlying concerns.
No, masturbation does not cause PCOS (Polycystic Ovary Syndrome). PCOS is a hormonal disorder linked to genetics, lifestyle, and other factors. There is no connection between PCOS and masturbation.
Masturbation can temporarily influence hormones like oxytocin and dopamine, but it does not cause long-term hormonal changes or imbalances.
There is no evidence that masturbation significantly increases estrogen levels. Hormonal shifts during masturbation are brief and do not impact overall estrogen levels.
No, masturbation does not affect implantation. If you are trying to conceive, masturbation will not interfere with the process of embryo implantation in the uterus.
While masturbation does not directly increase fertility, it can help reduce stress, which is beneficial for overall reproductive health.
There is no set frequency that is “right” or “wrong.” It relies on individual preferences and comfort levels. As long as it does not interfere with daily life or relationships, masturbation is perfectly healthy.
Hot baths rarely cause permanent infertility, but prolonged or repeated exposure to high temperatures can lead to long-term damage in severe cases. It best to avoid regular hot baths if you are trying to conceive.
Yes, cold baths may support fertility by helping maintain a healthy scrotal temperature. Unlike prolonged heat exposure, which can harm sperm quality, cooler temperatures promote optimal sperm production.
Yes, taking a bath after trying to conceive is generally safe, as long as the water is not too hot. Avoid hot tubs or saunas, as high temperatures may temporarily reduce sperm quality.
Steam baths can promote relaxation and improve circulation. However, prolonged heat exposure can affect sperm quality temporarily. To maintain reproductive health, opt for lukewarm baths limited to 10–15 minutes.
Some effective self-care practices during IUI/IVF treatment include:
- Engaging in exercise, such as yoga, walking, or light cardio
- Practicing relaxation techniques
- Getting enough sleep and rest
- Having a balanced, nutrient-rich diet
Yes, seeking counselling can be incredibly beneficial in managing the emotional stress of undergoing IUI/IVF alone. A mental health professional can provide a safe and supportive space for you to process your feelings, develop coping strategies, and learn techniques to maintain your emotional well-being throughout the treatment process.
To stay proactive about your treatment while your partner is away, consider the following strategies:
- Familiarise yourself with the details of your treatment plan
- Maintain regular communication with your team of doctors
- Utilise technology, such as fertility tracking apps and video calls, to keep your partner informed
- Create a detailed treatment checklist
Fertility alarms like the Xiltrix Alarm System can be highly customisable to accommodate the unique requirements of different fertility treatments and laboratory environments. Clinics can work with the system’s manufacturer to configure the monitoring parameters, alert thresholds, and notification protocols to align with their specific needs and safety protocols.
IUI చికిత్స తర్వాత తేలికపాటి శారీరక శ్రమ ఆమోదయోగ్యమైనది. రోగులు మొదటి వారం వ్యాయామాన్ని సున్నితమైన నడక మరియు సాగదీయడానికి పరిమితం చేయాలి. అధిక-ప్రభావ కార్యకలాపాలు మరియు బరువులు ఎత్తడం మానుకోండి, ఎందుకంటే ఇవి ఇంప్లాంటేషన్కు ఆటంకం కలిగిస్తాయి.
సమతుల్య, పోషకమైన ఆహారం గర్భధారణకు సరైన పరిస్థితులకు మద్దతు ఇస్తుంది. వీటిపై దృష్టి పెట్టండి:
- తాజా పండ్లు మరియు కూరగాయలు
- లీన్ ప్రోటీన్లు
- తృణధాన్యాలు
- ఫోలిక్ యాసిడ్ అధికంగా ఉండే ఆహారాలు
- తగినంత నీరు త్రాగడం
IUI తర్వాత నివారించాల్సిన సాధారణ ఆహారాలు:
- కెఫిన్
- ఆల్కహాలిక్ పానీయాలు
- స్వోర్డ్ ఫిష్ మరియు ట్యూనా వంటి అధిక పాదరసం కలిగిన చేపలు
- ముడి/ఉడికించని మాంసాలు లేదా గుడ్లు
- ప్రాసెస్ చేసిన ఆహారాలు
- చక్కెర స్నాక్స్
IUI తర్వాత స్వల్ప దూర ప్రయాణం సాధారణంగా సురక్షితమే, కానీ సుదూర ప్రయాణాలను వాయిదా వేసుకోవాలి. రోగులు తమ వైద్యుడితో ప్రయాణ ప్రణాళికలను చర్చించి, వారి గమ్యస్థానంలో వైద్య సంరక్షణ పొందుతున్నారని నిర్ధారించుకోవాలి.
రోగులు ఏదైనా మందులు తీసుకునే ముందు వారి సంతానోత్పత్తి నిపుణులను సంప్రదించాలి. ఇంప్లాంటేషన్తో సంభావ్య జోక్యాన్ని నివారించడానికి IUI తర్వాత కాలంలో సూచించిన మందులు మాత్రమే సిఫార్సు చేయబడతాయి.
గర్భధారణ పరీక్ష కోసం సిఫార్సు చేయబడిన వేచి ఉండే కాలం IUI ప్రక్రియ తర్వాత 14 రోజులు. చాలా త్వరగా పరీక్షించడం వల్ల తప్పుడు ఫలితాలు మరియు అనవసరమైన ఆందోళనకు దారితీయవచ్చు.
IUI తర్వాత కాలంలో మద్యం సేవించడం పూర్తిగా మానేయాలని సిఫార్సు చేయబడింది. మద్యం సేవించడం వల్ల విజయవంతమైన ఇంప్లాంటేషన్ అవకాశాలు తగ్గుతాయి మరియు గర్భధారణ ప్రారంభంలోనే ప్రభావితమవుతాయి.
ధూమపానం IUI విజయ రేటును గణనీయంగా తగ్గిస్తుంది మరియు పూర్తిగా మానుకోవాలి. ఇందులో చురుకైన ధూమపానం మరియు చికిత్స సమయంలో సెకండ్హ్యాండ్ పొగకు గురికావడం రెండూ ఉంటాయి.
Light physical activity is acceptable after IUI treatment. Patients should limit exercise to gentle walking and stretching for the first week. Avoid high-impact activities and heavy lifting that could interfere with implantation.
A balanced, nutritious diet supports optimal conditions for conception. Focus on fresh fruits and vegetables, lean proteins, whole grains, foods rich in folic acid, and adequate water intake. Common foods to avoid include caffeine, alcoholic beverages, high-mercury fish (like swordfish and tuna), raw or undercooked meats or eggs, processed foods, and sugary snacks.
Short-distance travel is generally safe after IUI, but long journeys should be postponed. Patients should discuss any travel plans with their doctor and ensure access to medical care at their destination.
Patients should consult their fertility experts before taking any medication. Only prescribed medications are recommended during the post-IUI period to avoid potential interference with implantation.
The recommended waiting period for a pregnancy test is 14 days after the IUI procedure. Testing too early may lead to false results and unnecessary anxiety.
Complete abstinence from alcohol is recommended during the post-IUI period. Alcohol consumption can reduce the chances of successful implantation and affect early pregnancy.
Smoking reduces IUI success rates significantly and should be avoided completely. This includes both active smoking and exposure to secondhand smoke during the treatment period.
Sperm washing is the process of isolating the sperm from the seminal fluid and other components of the semen sample, often used in assisted reproductive techniques like IUI. On the other hand, semen analysis is a diagnostic test that evaluates the characteristics of the semen, such as sperm count, motility, and morphology, to assess male fertility.
Men trying to conceive with their partner should undergo a semen analysis as part of the initial fertility evaluation. Depending on the results and treatment progress, follow-up tests may be recommended periodically to monitor sperm health and guide fertility strategies.
A semen analysis test typically assesses several factors: sperm count (number of sperm), sperm motility (movement ability), sperm morphology (shape and structure), semen volume, and pH levels. These parameters provide crucial insights into male fertility potential and help in determining appropriate treatments.
Sperm washing is generally a safe and commonly used procedure in fertility treatments. However, there is a minimal risk that the sperm may be damaged during the process, particularly during centrifugation. When performed by skilled professionals, the risk is significantly reduced, and the quality of the sperm sample is maintained.
The IUI (Intrauterine Insemination) procedure is usually performed shortly after sperm washing—typically within a few hours. This timing ensures that the sperm sample is at its highest quality and most motile state for optimal chances of fertilisation during insemination.
తీసుకోవచ్చు, కానీ మీకు ఐరన్ లోపం ఉంటే మాత్రమే. ఐరన్ సప్లిమెంట్లు తీసుకోవడం వల్ల అండోత్పత్తికి సంబంధించిన సమస్యలు పరిష్కారమవడమే కాకుండా, మీరు గర్భవతి అయిన తర్వాత రక్తహీనత రాకుండా నివారించవచ్చు. అయితే, ఎలాంటి సప్లిమెంట్లు మొదలుపెట్టే ముందైనా తప్పనిసరిగా మీ డాక్టర్ను సంప్రదించడం ముఖ్యం.
అవును, సంతాన సామర్థ్యం మరియు గర్భస్థ శిశువు ఆరోగ్యం రెండింటినీ ప్రభావితం చేసే ముఖ్య పోషకాలలో ఐరన్ ఒకటి. ఐరన్ లోపం వల్ల తల్లులలో అనారోగ్య సమస్యలు పెరగడం, గర్భస్థ శిశువు మరణాలు మరియు ప్రసవ సమయంలో సమస్యలు వంటి తీవ్రమైన పరిణామాలు ఏర్పడవచ్చు. ఆరోగ్యకరమైన గర్భధారణ కోసం ఐరన్ స్థాయిలను సరైన మోతాదులో ఉంచుకోవడం చాలా ముఖ్యం.
ఐరన్ లోపం వల్ల ఋతుచక్రాలు సరిగా రాకపోవడం లేదా ఆలస్యం కావడం జరగవచ్చు. మరింత తీవ్రమైన సందర్భాల్లో, అధిక రక్తస్రావాన్ని నివారించడానికి శరీరం ఒక రక్షణ చర్యగా నెలసరిని పూర్తిగా ఆపివేయవచ్చు.
ఐరన్ లోపాన్ని సాధారణంగా మహిళల సంతాన సామర్థ్యంతో ముడిపెట్టి చూసినప్పటికీ, ఇది పురుషుల సంతాన సామర్థ్యంపై కూడా ప్రభావం చూపుతుంది. ఐరన్ స్థాయిలు తక్కువగా ఉండటం వీర్య కణాల సంఖ్య మరియు పనితీరుపై ప్రభావం చూపుతుంది. దీనివల్ల వీర్య కణం అండాన్ని విజయవంతంగా ఫలదీకరణం చేయడం కష్టమవుతుంది.
There is no universal rule. Masturbation is considered healthy as long as it doesn’t interfere with your daily life, relationships, or responsibilities. Everyone has a different level of comfort and frequency, and what’s healthy varies from person to person.
No, masturbation does not affect menstrual cycles. However, it may help relieve menstrual cramps, reduce stress, and improve mood during your period by releasing feel-good hormones like endorphins.
Yes, masturbation is generally safe during pregnancy unless advised otherwise by a healthcare provider. It can help reduce stress, improve sleep, and promote overall well-being. However, its important to avoid lubricants or toys containing spermicide, as spermicide may be harmful to the developing fetus.
The frequency of masturbation that is considered “too often” varies from person to person. It may be a concern if it begins to negatively impact your physical health, emotional well-being, social life, or daily responsibilities. In such cases, speaking to a therapist or counselor can help address any underlying issues and develop a balanced approach.
Following intrauterine insemination (IUI), it is usually advised to take a 20–30 minute rest. This increases the likelihood of successful fertilization by giving the injected sperm more time to reach the fallopian tubes.
Until your doctor gives the all-clear, avoid using saunas, hot tubs, and hot baths. It is advisable to be cautious since heat might disrupt the implantation process.
If you experience any unusual symptoms such as severe pain or persistent bleeding, consult your doctor immediately. Prompt treatment is important.
While IUI is generally safe, there are minor risks involved such as spotting, infection, or multiple pregnancies in some cases.
Like any medical procedure, ICSI carries some potential risks, including:
- Ovarian hyperstimulation syndrome (OHSS)
- Multiple pregnancies (in the case when more than one embryo is transferred)
- Ectopic pregnancy
However, these risks are generally low, and your fertility specialist will take appropriate measures to minimise them.
The timeline for seeing results after an ICSI procedure can vary. If a fresh embryo transfer is performed, you may take a pregnancy test approximately two weeks after the embryo transfer. If the transfer is successful, you will continue with prenatal care and monitoring.
If embryos are frozen for future use, the timeline will depend on when you choose to proceed with a frozen embryo transfer cycle.
The cost of ICSI treatment can vary significantly and depends on numerous factors, such as your location, the fertility clinic, and the extent of treatment required. ICSI is generally more expensive than traditional IVF due to the additional laboratory procedures involved.
The total cost may include fees for consultations, medications, egg retrieval, ICSI procedure, embryo transfer, and any additional testing or procedures required. Some clinics may offer package pricing or financing options to help manage the costs.
Yes, ICSI is particularly beneficial for couples facing severe male factor infertility issues, such as:
- Extremely low sperm count
- Poor sperm motility
- Abnormal sperm morphology
- Obstructive azoospermia
If ICSI is not successful after multiple attempts, there are alternative approaches that couples may consider, depending on their specific circumstances and preferences:
- Donor sperm or donor eggs: Using donor sperm or donor eggs can be an option for couples facing severe infertility issues.
- Gestational surrogacy: In cases where the female partner is unable to carry a pregnancy, gestational surrogacy may be an option, where another woman carries the pregnancy for the intended parents.
- Adoption: Adopting may be a viable path to building a family for some couples.
Lack of sleep creates more stress hormones in the body, which may interfere with estrogen, testosterone, and other reproductive hormone levels and be detrimental to general health. Sleep difficulties have been connected to irregular menstruation, ovulatory dysfunction, and decreased fertility in women. Ensuring proper sleep hygiene is vital when trying to conceive.
Diet and lifestyle greatly influence fertility. Numerous essential nutrients assist in maintaining the health of your reproductive system. A diet rich in whole grains, vegetables, seafood, and unsaturated fats is generally associated with increased fertility in both men and women. Having a balanced and nutrient-dense diet helps enhance fertility and overall reproductive health.
Yes, certain jobs can affect fertility more than others. Women who work in jobs that require shift work or involve heavy lifting may experience greater difficulty conceiving. These physical and schedule-related demands can disrupt hormonal balance and ovulation, leading to decreased fertility compared to women with less physically demanding jobs and more regular hours.
Job satisfaction is closely related to mental and emotional well-being. Satisfied employees typically experience lower levels of stress and anxiety, which can positively affect hormone regulation and reproductive health. High job satisfaction may contribute to better fertility outcomes by reducing chronic stress and its negative impact on the reproductive system.
For women in their mid to late-20s, freezing 15–20 eggs is generally recommended to increase the chances of achieving a successful pregnancy in the future. This number provides a good probability of having viable eggs when you are ready to conceive later in life.
Yes, women with medical conditions like cancer or autoimmune diseases can consider egg freezing before undergoing treatments that may impact fertility. It offers a way to preserve reproductive potential ahead of procedures like chemotherapy or radiation.
Most women in their mid to late-20s typically require one or two cycles of ovarian stimulation to retrieve enough eggs for freezing. The exact number can vary based on individual ovarian reserve and response to treatment.
Several groups of people can benefit from egg freezing for future use. These include women experiencing age-related fertility decline, those undergoing fertility-damaging medical treatments, women focusing on education or career goals, individuals with PCOS or health concerns, those delaying marriage or parenthood, and individuals with genetic concerns.
Yes, if you are facing medical treatments like chemotherapy or radiation that may harm your fertility, you can preserve your eggs through freezing and use them later when you’re ready to conceive.
While there is no strict upper age limit for egg freezing, the best time to freeze eggs is in your 20s to early 30s. This is when egg quality and quantity are at their peak, which increases the chances of successful fertilisation later.
The egg freezing process at Ferty9 involves five steps: consultation with a fertility expert, ovarian stimulation to produce multiple eggs, egg retrieval through a minor surgical procedure, vitrification (freezing), and secure storage until you’re ready to use them.
Egg freezing is generally not painful, though some discomfort may occur. You might experience mild cramping during certain stages, and hormonal injections may cause slight stinging. A nurse will assist you with the process to ensure minimal discomfort.
Eggs can typically be stored for up to 10 years, depending on country regulations and clinic policies. Some clinics may allow longer storage, but it’s important to note that the quality of frozen eggs may decline over extended periods.
The cost of egg freezing at Ferty9 varies based on factors like the clinic location, medical team expertise, procedures involved, and any additional services or medications. For accurate pricing, it is recommended to consult a specialist at Ferty9 directly.
Egg freezing involves retrieving mature eggs from the ovaries, selecting and grading them, then freezing them using vitrification. These frozen eggs are then stored securely and can be used for future fertility treatments when you’re ready.
No, fertility typically returns shortly after stopping oral contraceptives. Most women can conceive within a few months of discontinuation.
The relationship between contraceptive pills and weight gain is a common concern. Some women may experience a slight increase in weight due to fluid retention, but studies generally show that modern contraceptive pills are not significantly associated with long-term weight gain. Individual responses can vary, and factors like lifestyle, genetics, and metabolism also play a role. Women need to discuss potential side effects with their healthcare provider when choosing contraceptive methods.
Birth control pills can be safe and effective for teenagers. However, the decision to start using contraceptives should consider factors like the teenagers medical history, lifestyle, and sexual activity. Healthcare providers can give guidance on the most suitable options for contraception, discuss potential side effects, and ensure proper usage to maximize effectiveness and safety.
Recurrent pregnancy loss is not the same thing as infertility. While repeated pregnancy loss (RPL) may not directly influence fertility, it can induce mental discomfort and worry, which may have an indirect impact on a couple’s attempt to conceive. Seeking adequate medical and emotional support is critical for overcoming the problems of recurrent loss and achieving fertility treatment objectives, especially in addressing infertility problems in females.
A woman suffering from fallopian tube obstruction may have difficulty conceiving. Women with obstructed fallopian tubes may have symptoms such as discomfort in the pelvis or abdomen, as well as pain throughout their menstrual cycle.
Weight can disturb hormone balance, resulting in reduced fertility. The amount and distribution of body fat influence the menstrual cycle via a variety of hormonal pathways. The more body weight and belly fat, the higher the chance of infertility.
High cortisol levels can disrupt hormone balance, especially for reproductive hormones like progesterone and estrogen. An imbalance in hormones may disrupt ovulation and menstrual cycles, making conception more challenging. Stress can also cause unusual menstrual periods.
Surgeries are unlikely to have an impact on fertility and often do not interact with the reproductive organs. But scar tissue, or adhesions, resulting from any type of surgery can obstruct the fallopian tubes, which can cause infertility.
The sperm DNA fragmentation test is generally reliable; however, its accuracy depends on the method used—such as the TUNEL assay, SCSA, or COMET assay—as well as the quality of the semen sample. Each testing method has its own sensitivity and specificity, which can influence the results.
A sperm DNA fragmentation index (DFI) of 15% or less is considered normal. DFI values between 15% and 30% are regarded as moderate or average, while values above 30% are considered high and may significantly impact fertility. It is advisable to consult a fertility expert for interpretation and next steps.
Sperm DNA fragmentation cannot be completely reversed, but its effects can often be mitigated. Lifestyle modifications, such as quitting smoking, improving diet, reducing stress, and taking antioxidant supplements, can help. Additionally, treating underlying conditions like varicocele or infections can improve sperm quality over time.
High levels of sperm DNA fragmentation can negatively impact IVF outcomes by reducing embryo quality, lowering implantation rates, and increasing miscarriage risks. Addressing high fragmentation levels before IVF can improve the likelihood of a successful pregnancy.
Vaginismus rarely resolves on its own, as it is frequently associated with psychological, emotional, or physical causes. However, with the right treatment, including pelvic floor therapy, psychotherapy, and relaxation techniques, many people may overcome it. Seeking expert care can significantly improve symptoms and overall quality of life.
Yes, a woman with vaginismus can conceive naturally if penetration is possible or through alternative methods like intrauterine insemination (IUI) or assisted reproductive techniques.
The duration of vaginismus treatment varies according to the severity of the condition and the patients response to treatment. Some women show relief within a few weeks with frequent pelvic floor therapy and relaxation exercises, while others may need many months.
When a man or woman is diagnosed with sterility, their reproductive potential is completely lost. Unlike infertility, which may have treatment options, sterility typically cannot be reversed. In most cases, the condition is permanent, and natural conception is not possible.
Sterility is less common compared to infertility. While infertility affects a significant number of couples and often has treatable causes, sterility refers to a complete inability to conceive and is much rarer.
Stress alone is unlikely to cause sterility, but it can significantly impact fertility. Chronic stress can disrupt hormonal balance and interfere with ovulation. Studies show that women with a history of depression are twice as likely to face infertility, and anxiety can also delay conception.
Age is one of the most critical factors influencing fertility and the risk of sterility. A woman’s fertility starts to decline around age 30 and drops more sharply after the mid-30s. As age increases, not only do the chances of conception decrease, but the likelihood of complications and permanent reproductive challenges also rises.
The social stigma surrounding infertility in India can have significant psychological and emotional consequences for affected couples. Many individuals and couples face societal pressure, discrimination, and even isolation due to the cultural emphasis on having children. This stigma can discourage open discussions and seeking timely medical assistance, exacerbating the challenges of infertility.
Several misconceptions surround infertility, such as the belief that it is solely a female issue, that stress is the primary cause, or that fertility treatments are always successful. It’s important to dispel these myths and promote accurate information to ensure couples receive appropriate support and treatment.
Urbanisation has been linked to a decline in fertility rates in India. Factors such as increased education levels, career aspirations, delayed marriages, and the high cost of living in urban areas can contribute to couples postponing or limiting their families.
Some common fertility myths in Indian society include beliefs that certain foods or practices can improve fertility, that infertility is a punishment for past misdeeds, or that traditional remedies can cure infertility without medical intervention. These myths can be harmful and prevent individuals from seeking appropriate medical care.
Stress can exacerbate infertility by altering hormonal balance and ovulation, especially in women. Chronic stress may impair sperm quality and count in males, affecting conception. High stress levels can also lead to unhealthy lifestyle habits, which further contribute to fertility challenges. Managing stress through mindfulness, therapy, or lifestyle changes can positively impact reproductive health.
Early indicators of infertility include irregular or nonexistent menstrual periods, which may signal ovulation issues in women. In men, indications like diminished libido, pain during ejaculation, or a notable drop in sperm quality can signal probable fertility concerns. Identifying these signs early and consulting a fertility expert can help in timely diagnosis and treatment.
Yes, lifestyle modifications such as eating a balanced diet, exercising regularly, limiting alcohol and tobacco consumption, and managing stress can boost fertility. Optimizing weight, avoiding pollutants, and getting enough sleep can enhance reproductive health for both men and women. Making these adjustments not only supports conception but also promotes overall well-being.
Yes, lifestyle choices can significantly impact male fertility. Factors such as excessive alcohol intake, smoking tobacco, poor diet, lack of physical activities, and exposure to environmental toxins or radiation can negatively affect sperm count, motility, and overall sperm quality. Making positive lifestyle changes can often improve male fertility.
Most fertility tests for men are non-invasive and carry minimal risks. However, procedures like testicular biopsy may involve minor discomfort or bruising. Your doctor will take you through the potential risks or side effects associated with specific tests and guide you in managing them.
Anti-sperm antibody testing helps detect the presence of antibodies that may attack and immobilise sperm, hindering fertilisation. This test typically involves collecting a semen sample and analysing it for antibodies that bind to sperm. In some cases, doctors may also conduct a blood test to check for anti-sperm antibodies.
The success rate of male infertility treatments can vary significantly and depends on the underlying cause and the specific treatment approach. With advances in assisted reproductive technologies (ARTs), such as intracytoplasmic sperm injection (ICSI), many couples have successfully conceived despite male infertility factors. However, it’s important to discuss your chances of success with your fertility specialist based on your circumstances.
Poor ovarian reserve means a reduced quantity or quality of a woman’s remaining eggs, often impacting fertility and response to treatment. It may lead to challenges in conceiving naturally or responding adequately to fertility treatments.
The Anti-Müllerian Hormone (AMH) test is considered the most reliable method for assessing ovarian reserve. It measures hormone levels linked to egg production and can be done at any time during the menstrual cycle.
While poor ovarian reserve cannot be completely reversed, there are effective treatment options available. These include fertility medications, in vitro fertilization (IVF), and the use of donor eggs. With the right treatment plan, many women can achieve successful pregnancies.
Ovarian reserve naturally declines with age. Both the quantity and quality of eggs decrease over time, with a more rapid decline occurring after the age of 35. This impacts fertility and the likelihood of a successful pregnancy.
Yes, it is possible to conceive naturally with diminished ovarian reserve, especially in younger women and those without additional fertility issues. However, early evaluation and medical guidance are important to maximize the chances of natural conception.
Balancing hormones to improve fertility varies from person to person. The time required may range from a few weeks to several months, depending on the type and severity of the imbalance, underlying health conditions, lifestyle factors, and medical treatment plans.
Common symptoms of hormonal imbalances in female infertility include irregular or heavy periods, loss of libido, vaginal dryness, hot flashes, hair loss, acne, and vaginal atrophy. These signs may vary in intensity based on the individual’s health and hormone levels.
A variety of tests are used to diagnose hormonal imbalances, including blood hormone panels, anti-Mullerian hormone tests, thyroid-stimulating hormone (TSH) levels, prolactin levels, testosterone and DHEA-sulfate levels, insulin and glucose testing, pelvic ultrasound, Hysterosalpingogram (HSG), Progesterone Challenge Test, and Genetic Testing.
Hormonal imbalances can disrupt the development and release of healthy eggs, impact the timing and regularity of ovulation, and lead to irregular or missed menstrual cycles. This interference can significantly reduce the chances of conception.
Yes, in most cases, hormonal imbalances related to infertility are reversible. Once the underlying cause is identified, fertility specialists can provide treatments that restore hormonal balance and improve the chances of conception.
Yes, dietary and lifestyle changes can play a significant role in correcting hormonal imbalances. A well-balanced diet, regular exercise, weight management, and reduced stress levels can help restore hormonal health and enhance fertility naturally.
Depending on patient response and clinic procedures, the ICSI procedure usually takes four to six weeks from ovarian stimulation to embryo transfer.
Yes, ICSI is an effective way to treat unexplained male infertility conditions, especially those that involve low sperm counts, poor sperm motility, or sperm with unusual shapes that might make it difficult for them to naturally fertilize an egg.
After a comprehensive evaluation that includes hormone testing, semen analysis, ovulation tracking, and imaging of the reproductive organs, unexplained infertility is diagnosed when no obvious cause is identified despite normal results.
Unexplained female infertility can be caused by undiagnosed problems with egg quality or release, modest hormone imbalances, or reproductive system abnormalities that are not detectable by routine testing.
Age is a major factor in unexplained infertility because female fertility decreases with age, especially after the age of 35, as a result of decreased egg quality and quantity. Even in cases where there are no other obvious causes of infertility, this may result in problems conceiving. As men age, their sperm quality may also decline, which adds to their infertility problems.
If small amounts of scar tissue or adhesions obstruct your fallopian tubes, your doctor can perform laparoscopic surgery to remove the obstruction and open them. However, if the blockage is severe and caused by extensive scar tissue or adhesions, it may not be possible to remove it through laparoscopy. In such cases, other fertility treatment options may need to be considered.
The typical recovery period following laparoscopic surgery is between 2 and 4 weeks, although this can vary depending on your specific condition. It is important to consult with your doctor before trying to conceive. Planning a pregnancy should only be done once your body has fully healed from the procedure and your doctor confirms it is safe to proceed.
Before undergoing laparoscopic surgery, you should inform your doctor about any prescription or over-the-counter medications you are taking. Your doctor will guide you on how to manage them before and after the procedure. Typically, you will need to avoid eating or drinking for at least 8 hours before surgery. Since the procedure is often performed under general anesthesia, it’s also advisable to have a family member or friend accompany you, as you may feel drowsy afterward.
During your first session, your counsellor will take the time to understand your unique condition, concerns, and goals. They will provide you with a safe and non-judgmental space to express your feelings and emotions. Together, you will explore coping strategies, decision-making processes, and potential next steps in your fertility journey.
Infertility counsellors may use various techniques, such as cognitive-behavioural therapy (CBT), mindfulness-based interventions, and couples counselling. The methods will depend on your individual needs and the counsellor’s approach.
Counselling can provide numerous emotional benefits for couples dealing with fertility problems, including:
- Reduced stress and anxiety
- Improved communication and intimacy
- Increased feelings of empowerment and control
- Healthy coping mechanisms for grief and disappointment
- A sense of support and understanding
Yes, counselling can be highly beneficial for couples experiencing relationship conflicts related to infertility. A counsellor can help you and your spouse communicate more effectively, navigate disagreements, and strengthen your emotional connection during this challenging time.
The frequency of counselling sessions will depend on your individual needs and the counsellor’s recommendations. Some couples may benefit from weekly or bi-weekly sessions, while others only need occasional check-ins. Your counsellor will work with you to determine the appropriate schedule.
Yes, there are specific lifestyle changes that both women and men should make to improve their fertility as a couple. These include regular exercise, maintaining a healthy weight, and consuming a balanced diet rich in fruits, lentils, and dairy. These changes promote hormonal balance and reproductive health, thereby improving the chances of conception.
Supporting your partner through IVF involves being emotionally available and physically present. Simple gestures like holding hands, listening empathetically, and being involved in appointments can make a big difference. Encouraging open conversations and seeking professional fertility counseling can also provide added support during this challenging journey.
Infertile couples may encounter various challenges, including emotional stress, depression, and social stigma. Relationship strains, such as misunderstandings or even divorce, can occur. In some cases, women may face violence, anxiety, or low self-esteem. It’s crucial for couples to seek support and communicate openly to cope with these difficulties together.
Comforting your wife after a failed IVF cycle involves emotional support and compassion. Acknowledge her feelings, offer validation, and create a safe space for her to express herself. Allow her time to grieve and process the experience. Encourage her to seek professional help if needed, and assure her that you’re in this journey together.
అవును, కొన్నిసార్లు అదనపు మందుల ఖర్చులు, నిర్ధారణ పరీక్షల ఖర్చులు, పిండాలను భద్రపరిచే (ఎంబ్రియో ఫ్రీజింగ్) ఖర్చులు, మరియు ఇక్సీ (ICSI) వంటి మరింత క్లిష్టమైన ప్రక్రియలకు అయ్యే ఖర్చులు ఐవిఎఫ్ చికిత్సలో దాగి ఉండే ఖర్చులకు ఉదాహరణలు. ఊహించని ఖర్చులను నివారించడానికి, చికిత్సకు అయ్యే పూర్తి ఖర్చుల వివరాలను ముందుగానే తెలుసుకోవడం చాలా ముఖ్యం. అయితే, మా ఫెర్టీ9 ఫెర్టిలిటీ సెంటర్లో చికిత్సలకు ఎలాంటి దాగి ఉన్న ఖర్చులు ఉండవని మీరు నిశ్చింతగా ఉండవచ్చు.
అవును, వయస్సు సంబంధిత అంశాలు తిరుపతిలో ఐవిఎఫ్ ఖర్చులను ప్రభావితం చేస్తాయి. ఎందుకంటే, వయసు పైబడిన రోగులకు మరింత ఆధునాతన చికిత్సలు, అదనపు మందులు, లేదా ఎక్కువ సైకిల్స్ అవసరం కావచ్చు. ఇవి మొత్తం ఖర్చులను పెంచుతాయి.
తిరుపతిలో ఐవిఎఫ్ ఖర్చుపై చికిత్సా నాణ్యత ఎంతో ప్రభావం చూపుతుంది. ఎందుకంటే, అత్యాధునిక సాంకేతికతను ఉపయోగించే మరియు అధిక సక్సెస్ రేట్లు ఉన్న క్లినిక్లు వారి సేవలకు ఎక్కువ రుసుము వసూలు చేయవచ్చు. ప్రతి వ్యక్తికి ప్రత్యేకమైన చికిత్సా ప్రణాళికలు మరియు రోగికి అందించే విస్తృతమైన సహాయం కూడా అధిక ఖర్చులకు దారితీయవచ్చు, కానీ అవి మొత్తం ఫలితాలను మెరుగుపరుస్తాయి. ఫెర్టీ9 తిరుపతి, అత్యాధునిక టెక్నాలజీ, అనుభవజ్ఞులైన నిపుణులు, విస్తృతమైన సేవలు, సరసమైన ధరలు, మరియు అన్నింటికంటే ముఖ్యంగా సహాయకరమైన వాతావరణంతో నాణ్యమైన సంరక్షణను అందిస్తుంది.
ఐవిఎఫ్ చికిత్సలో సమస్యలు తలెత్తినప్పుడు చికిత్సా కాలం పెరగవచ్చు, ఎక్కువ వైద్య జోక్యాలు (Medical Interventions) అవసరం కావచ్చు, మరియు పర్యవేక్షణ కూడా ఎక్కువ కావాలి. ఇవన్నీ మొత్తం ఖర్చులను పెంచుతాయి.
The RI Witness System tracks a wide range of data throughout the IVF process, including the unique identification of samples (such as oocytes, sperm, and embryos), the exact timestamps of each procedure, the personnel involved, and any relevant notes or observations. This comprehensive data collection creates a detailed audit trail that an authorised personnel can access and review.
By implementing the RI Witness System, clinics can demonstrate their commitment to accuracy, security, and quality throughout the IVF process. Patients can feel more confident and assured after knowing their samples and materials are being meticulously tracked and verified. This reduces the risk of mix-ups and enhances their confidence in the treatment journey.
The RI Witness System’s improved tracking and communication features can help clinics provide patients with more transparent and accessible information about their treatment status. This enhanced communication, coupled with the system’s reliability and security, can foster a greater sense of partnership and collaboration between clinicians and patients, ultimately supporting them through the emotional and complex IVF journey.
Unlike traditional manual witnessing methods, which rely on human observation and record-keeping, the RI Witness System utilises automated identification and tracking technologies to eliminate the potential for human error. By automating the verification process, the system ensures a higher level of accuracy and consistency throughout the IVF treatment, providing a more robust and reliable approach to safeguarding patient samples and materials.
The RI Witness System’s barcode and RFID identification capabilities ensure that each patient’s samples and materials, including their embryos, are uniquely tracked and verified at every stage of the IVF process. This meticulous tracking, coupled with the system’s real-time data management and comprehensive audit trails, helps clinicians to confidently select the correct embryos for transfer, minimising the risk of mix-ups and improving the overall success of the IVF treatment.
Embryo quality is critical in IVF because high-quality embryos are more likely to implant and grow into a viable pregnancy. Developmental stage, morphology, and genetic health have a substantial impact on success rates.
Day 5 blastocyst transfers are preferred over day 3 transfers due to their advanced stage, increased implantation potential, and better synchronization with the uterine lining. They also enable more precise embryo selection and lower the chance of multiple pregnancies when fewer embryos are transferred.
Typically, one or two blastocysts are transplanted during IVF to balance the chances of pregnancy while reducing the risk of multiple pregnancies. To prevent problems, patients with high-quality embryos are generally advised to undergo a single embryo transfer (SET).
పిండం బదిలీ చేసిన తర్వాత ఇంటి వద్ద చేసుకునే ప్రెగ్నెన్సీ పరీక్ష కోసం కనీసం 9-14 రోజులు వేచి ఉండాలి. అయితే, ఫెర్టిలిటీ క్లినిక్లో చేసే రక్త పరీక్ష చాలా నమ్మదగిన ఫలితాలను ఇస్తుంది. ఇది తక్కువ మొత్తంలో ఉన్న ప్రెగ్నెన్సీ హార్మోన్లను కూడా గుర్తించగలదు.
మొదటి స్కాన్కు సిద్ధం కావడానికి కొన్ని ముఖ్యమైన విషయాలు:
– క్లినిక్ చెప్పినట్లుగా నీరు త్రాగాలి.
– సౌకర్యవంతమైన, వదులుగా ఉండే దుస్తులు వేసుకోవాలి.
– అవసరమైన అన్ని వైద్య పత్రాలు తీసుకురావాలి.
– వీలైతే ఉదయం వేళల్లో అపాయింట్మెంట్ పెట్టుకోవాలి.
– తేలికపాటి అల్పాహారం తీసుకోవాలి.
– కావాలనుకుంటే ఒక సహాయకుడిని వెంట తీసుకెళ్లవచ్చు.
మొదటి స్కాన్కు ముందు ఆందోళనగా ఉండటం చాలా సాధారణం మరియు చాలా మంది IVF రోగులు దీనిని అనుభవిస్తారు. వైద్యులు ఈ ఆందోళనలను అర్థం చేసుకుంటారు మరియు ప్రక్రియ అంతటా భరోసా మరియు మద్దతు ఇవ్వడానికి సిద్ధంగా ఉంటారు.
ప్రారంభ ప్రెగ్నెన్సీ సంకేతాలలో రొమ్ము సున్నితంగా ఉండటం, కొద్దిగా తిమ్మిరి, అలసట మరియు ఆకలిలో మార్పులు ఉండవచ్చు. అయితే, కొంతమంది రోగులకు ఎటువంటి లక్షణాలు ఉండకపోవచ్చు, ఇది గర్భధారణలో ఎటువంటి సమస్యలు లేవని సూచిస్తుంది.
పరీక్షకు ముందు శారీరకంగా సిద్ధం కావడం ఎంత ముఖ్యమో, మానసికంగా సిద్ధం కావడం కూడా అంతే ముఖ్యం. భార్యాభర్తలు తమ ఆందోళనల గురించి ఫెర్టిలిటీ బృందంతో చర్చించాలి, ముందు రోజు రాత్రి బాగా నిద్రపోవాలి మరియు ప్రశాంతంగా మరియు సానుకూలంగా ఉండటంపై దృష్టి పెట్టాలి. ముందుగా ప్రశ్నలు రాసుకోవడం వల్ల అపాయింట్మెంట్ను సద్వినియోగం చేసుకోవచ్చు.
పిండం ఆరోగ్యంగా పెరగడానికి మరియు శరీర భాగాలు (గుండె, మెదడు లాంటివి) సరిగ్గా తయారవ్వడానికి పుట్టుకతో వచ్చే లక్షణాలు (జన్యువులు), తల్లి ఆరోగ్యం మరియు మంచి ఆహారం చాలా ముఖ్యం. అంతేకాకుండా, గర్భవతిగా ఉన్నప్పుడు సిగరెట్ ఇంక మద్యం తాగకపోవడం, మరియు మత్తు పదార్థాలు వాడకపోవడం చాలా అవసరం. చుట్టూ ఉండే కాలుష్యం కూడా బిడ్డకు మంచిది కాదు. క్రమం తప్పకుండా డాక్టర్ను కలిసి పరీక్షలు చేయించుకుంటే ఏమైనా సమస్యలు ఉంటే ముందుగానే తెలుస్తాయి మరియు వాటిని సరిచేసుకోవచ్చు. దీనివల్ల గర్భం బాగా నిలబడుతుంది మరియు ఆరోగ్యకరమైన బిడ్డ పుడతాడు.
అవును, గర్భవతిగా ఉన్నప్పుడు ఎక్కువ ఒత్తిడి ఉంటే అది కడుపులో ఉన్న బిడ్డ ఆరోగ్యంపై చెడు ప్రభావం చూపుతుంది. చాలా ఒత్తిడి ఉంటే నెలలు నిండకుండానే ప్రసవం అయ్యే అవకాశం ఉంది (37 వారాల కంటే ముందే కాన్పు రావచ్చు). అంతేకాకుండా, పిండం బరువు కూడా తక్కువగా ఉండవచ్చు. ఎక్కువ కాలం ఒత్తిడిలో ఉంటే తల్లికి బీపీ పెరగడం, గుండె జబ్బులు రావడం వంటి సమస్యలు కూడా వస్తాయి. ఈ సమస్యలన్నీ పిండం ఎదుగుదలను కూడా అడ్డుకుంటాయి. అందుకే, గర్భవతిగా ఉన్నప్పుడు ఒత్తిడిని తగ్గించుకోవడానికి మంచి పనులు చేయడం, ఇంట్లో వాళ్ళు మరియు స్నేహితుల సహాయం తీసుకోవడం చాలా ముఖ్యం.
పిండం మరియు కడుపులో ఉన్న శిశువు ఆరోగ్యంగా ఎదగడానికి సరైన ఆహారం చాలా ముఖ్యం. మంచి పోషకాహారం వల్ల వారి శరీర భాగాలు సరిగ్గా తయారవుతాయి, వారు బాగా పెరుగుతారు మరియు వారి ఆరోగ్యం కూడా బాగుంటుంది. గర్భవతిగా ఉన్నప్పుడు ఫోలిక్ యాసిడ్, ఐరన్, కాల్షియం మరియు ఒమేగా-3 కొవ్వు ఆమ్లాలు వంటి పోషకాలు తప్పకుండా తీసుకోవాలి. సమతుల్యమైన మరియు పోషకాలు ఎక్కువగా ఉన్న ఆహారం తీసుకోవడం వల్ల పుట్టుకతో వచ్చే లోపాలు మరియు ఎదుగుదల సమస్యలు వచ్చే ప్రమాదం తగ్గుతుంది.
మొదటి మూడు నెలల గర్భధారణ సమయంలో కాలుష్యం, సీసం, పాదరసం వంటి భారీ లోహాలు, పంటలపై చల్లే మందులు మరియు రేడియేషన్ వంటి పర్యావరణంలోని హానికరమైన పదార్థాలు పిండం ఎదుగుదలపై చాలా చెడు ప్రభావం చూపుతాయి. ఈ మొదటి మూడు నెలల్లోనే బిడ్డ యొక్క ముఖ్యమైన భాగాలు (గుండె, మెదడు వంటివి) తయారవుతాయి. కాబట్టి, ఈ సమయంలో హానికరమైన పదార్థాలకు దూరంగా ఉంటే పుట్టుకతో వచ్చే లోపాలు, ఎదుగుదల ఆలస్యం మరియు ఇతర గర్భ సంబంధిత సమస్యలు రాకుండా ఉంటాయి. పిండం ఆరోగ్యంగా పెరగడానికి గర్భం మొదలైనప్పటి నుండి ఈ ప్రమాదాలను తగ్గించడం చాలా ముఖ్యం.
The embryo transfer procedure time is roughly about 15 minutes. With precision and ultrasound assistance, infertility professionals get the procedure done effectively.
The whole procedure is completely non-invasive, meaning there shouldn’t be any pain. Although some might say that embryo transfer is painful, it is a completely painless procedure, but women describe it as a sensation of mild discomfort.
Factors that can affect the success of embryo transfer include sperm quality, age, fertilization rate, and the embryo quality.
If these post-transfer symptoms like severe cramps or spotting occur, don’t panic. Call your doctor and seek advice.
After your embryo transfer, you are advised to wait for two weeks, which is also called a two-week wait time. After that, you can visit your clinic to test for pregnancy.
The 17th and 20th days of the cycle are considered best for it, and the frozen embryo transfer implantation timeline is also about 1-3 days.
You should take precautions like avoid lifting heavy objects, smoking, and alcohol. You should also take care of your medication time and cycle.
During the IVF cycle, implanting one or more embryos on the 17th and 20th day is considered effective for a successful conception.
It’s about 3 to 4 weeks after your recent period when you can expect the frozen embryo transfer.
గులాబీ రంగు చుక్కలు (స్పాటింగ్) ప్రారంభ గర్భధారణకు సంకేతం కావచ్చు, ముఖ్యంగా ఇది మీ పీరియడ్ రావలసిన సమయంలో సంభవిస్తే. ప్రారంభ గర్భధారణలో గులాబీ రంగు చుక్కలు (స్పాటింగ్) సంభవించినప్పటికీ, ఇది ఇతర కారకాల వల్ల కూడా కలగవచ్చని గమనించడం ముఖ్యం, కాబట్టి నిర్ధారణ కోసం ఆరోగ్య సంరక్షణ ప్రదాతను సంప్రదించడం మంచిది.
ఇంప్లాంటేషన్ రక్తస్రావం సాధారణంగా లేత గులాబీ లేదా గోధుమ రంగులో ఉంటుంది మరియు మీ అంచనా వేసిన పీరియడ్కు కొన్ని రోజుల ముందు సంభవిస్తుంది. ఇది సాధారణంగా సాధారణ పీరియడ్ కంటే తేలికగా ఉంటుంది మరియు తక్కువ వ్యవధి పాటు, సాధారణంగా 1-2 రోజులు ఉంటుంది. మీరు దీనిని అనుభవిస్తే, ఇది తరచుగా గర్భం యొక్క ప్రారంభ సంకేతం.
గర్భధారణ ప్రారంభంలో తేలికపాటి చుక్కలు (స్పాటింగ్) సాధారణం మరియు ఇది హార్మోన్ల మార్పులు లేదా ఇంప్లాంటేషన్ కారణంగా సంభవించవచ్చు. అయితే, పీరియడ్ మాదిరిగానే అధిక రక్తస్రావం వైద్య సహాయం అవసరం కావచ్చు, ఎందుకంటే ఇది గర్భస్రావం లేదా ఇతర గర్భధారణ సంబంధిత సమస్యలు వంటి సమస్యలను సూచించవచ్చు.
ఒక వారం పాటు గోధుమ రంగు పీరియడ్ బ్లడ్ సాధారణంగా శరీరం నుండి బహిష్కరించబడుతున్న పాత రక్తాన్ని సూచిస్తుంది. ఇది సాధారణం కావచ్చు, ముఖ్యంగా మీ పీరియడ్ ప్రారంభంలో లేదా చివరిలో. అయితే, ఏవైనా అంతర్లీన సమస్యలను తోసిపుచ్చడానికి దీర్ఘకాలిక గోధుమ రంగు రక్తస్రావం వైద్యుడిచే తనిఖీ చేయబడాలి.
ఐవీఎఫ్ గురించి ఆలోచించడానికి సరైన వయస్సు అనేది మీ ప్రాథమిక సంతానోత్పత్తి పరీక్షల (అంటే, మీ సంతాన సామర్థ్యాన్ని తెలుసుకోవడానికి చేసే ప్రాథమిక పరీక్షలు) ఫలితాలు మరియు ఇంతకు ముందు మీరు తీసుకున్న ఏవైనా సంతానోత్పత్తి చికిత్సల మీద ఆధారపడి ఉంటుంది. సంతానోత్పత్తి నిపుణుడిని (ఫెర్టిలిటీ స్పెషలిస్ట్) సంప్రదించడం వలన వారు మీకు వ్యక్తిగతంగా సలహా ఇవ్వగలరు మరియు మీకు ఏది ఉత్తమ సమయమో నిర్ణయించడానికి సహాయపడగలరు.
ఐవీఎఫ్ విజయంలో అండం నాణ్యత చాలా కీలకమైన పాత్ర పోషిస్తుంది. మంచి నాణ్యత కలిగిన అండాలు ఆరోగ్యకరమైన పిండాలుగా (ఎంబ్రియోలుగా) అభివృద్ధి చెందే అవకాశం ఎక్కువగా ఉంటుంది. దీనివల్ల, ఆ పిండం గర్భాశయంలో సరిగ్గా అతుక్కుని, గర్భం విజయవంతంగా నిలబడే అవకాశాలు పెరుగుతాయి.
Consuming between 1,000 and 2,000 mg of omega-3 fatty acids every day is recommended to increase fertility. Omega-3 fatty acids play a crucial role in the development and structure of all cells, including sperm and eggs, which are essential for successful conception.
Yes, omega-3 supplements may interact with certain medications, including cyclosporins, anticoagulants, and drugs that reduce blood pressure. It is essential to consult your healthcare practitioner about potential interactions between omega-3s and other medications or supplements before starting to take them.
Yes, omega-3 dietary supplements can be beneficial for individuals undergoing fertility treatments. They have been shown to improve fertilization rates and enhance embryo quality, thereby increasing the chances of pregnancy, particularly for women undergoing IVF treatments.
Yes, omega-3s can support fertility after a miscarriage or failed IVF cycle. They help by maintaining cell wall integrity, providing energy, and reducing the chances of complications such as preterm births, miscarriages, and stillbirths. Including omega-3s in your recovery plan may enhance reproductive health and improve future fertility outcomes.
కొన్నిసార్లు మీరు గర్భవతి కాకపోయినా ప్రెగ్నెన్సీ టెస్ట్లో పాజిటివ్ రావచ్చు. దీనికి కొన్ని ముఖ్యమైన కారణాలు ఉన్నాయి: కెమికల్ ప్రెగ్నెన్సీ (పిండం ఏర్పడినా, అది గర్భాశయానికి అతుక్కోకముందే ఆగిపోవడం), మందుల ప్రభావం (కొన్ని రకాల మందులు, ముఖ్యంగా సంతాన సాఫల్య చికిత్సలో వాడే HCG ఇంజెక్షన్లు తీసుకున్నప్పుడు), గర్భస్రావం జరిగిన వెంటనే టెస్ట్ చేయడం, టెస్ట్ స్ట్రిప్ను ఎక్కువ సేపు ఉంచి చదవడం, గడువు ముగిసిన టెస్ట్ కిట్ వాడటం వల్ల టెస్ట్ తప్పుగా పాజిటివ్ చూపించవచ్చు.
అవును, పీరియడ్స్ ఆలస్యమై, టెస్ట్ నెగటివ్గా వచ్చినా మీరు గర్భవతి అయ్యే అవకాశం ఉంది. దీనికి కారణం, మీ శరీరంలో ప్రెగ్నెన్సీని గుర్తించడానికి అవసరమైన హార్మోన్ (HCG) స్థాయిలు ఇంకా తక్కువగా ఉండవచ్చు. ముఖ్యంగా, మీరు పీరియడ్స్ రావాల్సిన తేదీకి ముందే లేదా కొద్ది రోజులకే టెస్ట్ చేసుకుంటే ఇలా జరగవచ్చు.
చాలా ప్రెగ్నెన్సీ టెస్టులు, పీరియడ్స్ రావాల్సిన తేదీ దాటిన మరుసటి రోజు చేసుకుంటే చాలా కచ్చితమైన ఫలితాన్ని ఇస్తాయి. అయితే, కొన్ని చాలా సున్నితమైన (highly sensitive) టెస్టులు అంతకంటే ముందుగానే, అంటే శరీరంలో ప్రెగ్నెన్సీ హార్మోన్లు (HCG) తక్కువ స్థాయిలో ఉన్నప్పుడే గర్భాన్ని గుర్తించగలవు. టెస్ట్ ఉదయం పూట, మొదటిసారి మూత్ర విసర్జన చేసినప్పుడు చేసుకోవడం మంచిది.
అవును, కొన్ని రకాల అనారోగ్య సమస్యలు ప్రెగ్నెన్సీ టెస్ట్ ఫలితాలను ప్రభావితం చేయవచ్చు. ఉదాహరణకు: కొన్ని రకాల అండాశయ తిత్తులు, పిట్యూటరీ గ్రంధికి సంబంధించిన సమస్యలు, కొన్ని అరుదైన కణితులు.
అత్యంత కచ్చితమైన ఫలితం కోసం, పీరియడ్స్ రావాల్సిన తేదీ దాటిన ఒకటి లేదా రెండు రోజుల తర్వాత టెస్ట్ చేసుకోవాలి. ఉదయం పూట చేసుకునే టెస్ట్, హార్మోన్ల స్థాయిలు ఎక్కువగా ఉండటం వలన అత్యంత నమ్మకమైన ఫలితాలను ఇస్తుంది. ఒకవేళ ఇంకా అనుమానంగా ఉంటే, కచ్చితమైన నిర్ధారణ కోసం పీరియడ్స్ ఆగిపోయిన ఒక వారం తర్వాత టెస్ట్ చేసుకోవాలని డాక్టర్లు సూచిస్తారు.
A fertility-friendly diet should focus on whole foods rich in antioxidants and essential nutrients. The most beneficial foods include leafy greens, nuts, seeds, fatty fish, and whole grains. These foods provide vital nutrients like folate, omega-3 fatty acids, and zinc that support reproductive health.
Research confirms that chronic stress can significantly impact fertility by disrupting hormone balance and menstrual cycles. Studies show that individuals with high-stress levels may take up to 29% longer to conceive compared to those with lower stress levels. Regular stress management is crucial for maintaining optimal fertility.
Most people begin to notice improvements in their reproductive health within three to four months of maintaining a consistent fertility diet. This timeframe allows for the complete cycle of egg and sperm development, making it the minimum period needed to see meaningful changes.
Fertility herbs can be safe when used properly under professional guidance. However, not all herbs suit everyone; some may interact with medications. Always consult an expert practitioner or ayurvedic doctor before starting any herbal supplements, especially if undergoing fertility treatments.
సంతానలేమిని ఇప్పుడు ఒక సామాజిక అపోహలా చూడటం లేదు, దానిపై ప్రజల అభిప్రాయాలు వేగంగా మారుతున్నాయి. ఒకప్పుడు సొంత రక్తం పంచుకు పుట్టిన పిల్లలకే అధిక ప్రాధాన్యత ఇచ్చిన భారతదేశంలో, ఇప్పుడు తల్లిదండ్రులుగా మారడానికి ఐవిఎఫ్ ఒక ఆమోదయోగ్యమైన మార్గంగా మారింది.
Essential fertility vitamins include:
- Folic acid (400–800 mcg daily)
- Vitamin D3 (2,000–3,000 IU)
- CoQ10 (200–600 mg)
- Omega-3 fatty acids (1,000–2,000 mg)
These nutrients support egg quality, hormone balance, and overall reproductive health.
ఐవిఎఫ్ సక్సెస్ రేటుపై వయస్సు గణనీయంగా ప్రభావం చూపుతుంది. తక్కువ వయస్సు ఉన్నవారిలో అండాల నాణ్యత, సంఖ్య మెరుగ్గా ఉండటం వల్ల విజయావకాశాలు ఎక్కువగా ఉంటాయి. అదే 35 ఏళ్లు దాటిన తర్వాత, ముఖ్యంగా 40 ఏళ్లు పైబడిన వారిలో సక్సెస్ రేట్లు తగ్గుతాయి.
సెలబ్రిటీలు తమ ఐవిఎఫ్ అనుభవాలను పంచుకోవడం వల్ల, సంతానలేమి గురించి మాట్లాడటం సమాజంలో మరింత ఆమోదయోగ్యంగా మారింది. ఈ బహిరంగ చర్చల ఫలితంగా, ఐవిఎఫ్ పట్ల పారదర్శకత పెరిగి, ఇప్పుడు దంపతులకు ఇది ఒక సాధారణ ఎంపికగా మారింది.
ఐవిఎఫ్ గురించి ఆలోచించేటప్పుడు, క్లినిక్ యొక్క పేరు (గౌరవం), దాని సక్సెస్ రేట్లు, మరియు అక్కడ ఉపయోగించే సాంకేతికతకు ప్రాధాన్యత ఇవ్వాలి. ఈ అంశాలు చికిత్స విజయవంతం అయ్యే అవకాశాలను గణనీయంగా ప్రభావితం చేస్తాయి. వీటితో పాటు మీ వయస్సు, గతంలో గర్భధారణ జరిగిన వివరాలు, జీవనశైలి మరియు మీ ఆరోగ్య చరిత్రను కూడా పరిగణనలోకి తీసుకోవడం ముఖ్యం.
At Ferty9, we understand the emotional devastation that can accompany a failed IVF cycle. Our counseling approach after a failed IVF cycle involves the following steps:
- Providing a safe space
- Exploring reasons for treatment failure
- Emotional support and discussing coping strategies
- Evaluating the readiness of the couple
- Discussing next steps
- Strengthening relationships among the couples
Getting started with Ferty9’s fertility counseling services is straightforward. Here are the steps you can take:
- Schedule an initial consultation
- Discuss your concerns
- Meet with a counselor
- Development of a personalized counseling plan
- Ongoing support throughout the journey
The ICSI process typically takes a few weeks to one or two months to complete, starting from the initial consultation and hormone treatments to egg retrieval and embryo transfer. While the exact duration can vary depending on individual circumstances, most ICSI cycles span approximately 4 to 6 weeks in total.
Ferty9’s fertility counseling services can be invaluable for individuals and couples in the pre-conception planning phase. During pre-conception counseling, our counselors can assist you with:
- Lifestyle evaluation
- Emotional preparation
- Relationship support
- Guidance on understanding your fertility cycles
- Exploring alternative family-building options
Common side effects of ICSI may include hormonal fluctuations, bloating, mood changes, and mild discomfort during the egg retrieval procedure. In rare cases, ovarian hyperstimulation syndrome (OHSS) may occur. These side effects are typically manageable, but it is important to report any unusual symptoms to a healthcare professional promptly.
If the ICSI cycle is unsuccessful, the fertility team will assess the outcome and help develop a tailored plan for future steps. This may involve modifying the treatment protocol, considering alternative fertility methods, or exploring options like donor gametes, surrogacy, or adoption, depending on the individual’s or couple’s preferences and medical circumstances.
Yes, certain lifestyle changes can enhance the likelihood of a successful ICSI outcome. These include maintaining a balanced and nutritious diet, engaging in regular moderate exercise, managing stress through relaxation techniques, avoiding smoking and excessive alcohol consumption, and addressing any existing medical conditions that may impact fertility.
Fertility drugs are generally safe when prescribed and monitored by a fertility specialist. Like any medication, they may have side effects, but your doctor will manage these to ensure your safety.
Yes. This is because fertility drugs can stimulate the ovaries to release multiple eggs during ovulation and increase the likelihood of multiple fertilizations.
Yes, fertility drugs help regulate hormone levels and stimulate ovulation, addressing one of the main causes of infertility in women with PCOS.
Research is ongoing, but potential long-term effects, like an increased risk of ovarian cancer, are under study. Your fertility specialist will discuss any concerns and monitor your health closely during treatment.
ICSI involves injecting a single sperm directly into an egg to facilitate fertilization, whereas regular IVF allows sperm to fertilize the egg naturally in a lab dish. ICSI is particularly beneficial for male infertility issues, such as low sperm count or poor motility.
The entire ICSI treatment process typically takes about four to six weeks, from the start of ovarian stimulation to the embryo transfer. The timeline can vary based on individual responses to treatment and any additional procedures required.
ICSI is particularly suitable for couples facing male infertility issues. However, it may not be necessary for all couples, especially those without significant sperm problems. A fertility specialist can help determine the most appropriate treatment based on individual circumstances.
To boost fertility in your 30s, focus on maintaining a healthy weight by eating a balanced diet, and managing stress. Incorporate moderate exercise and avoid smoking or excessive alcohol. Supplements like folic acid and antioxidants also support your reproductive health.
The timeline varies depending on individual factors, but many couples notice improvements within a few months of adopting these methods. Patience and consistency are the key when trying to boost fertility naturally.
Yes, foods rich in antioxidants, such as nuts, berries, and leafy greens, can protect eggs and sperm from oxidative stress. Whole grains, lean proteins, and healthy fats also support hormonal balance, making them excellent choices to boost fertility.
Emerging research suggests that incorporating the right types of physical activities into your lifestyle can positively impact fertility and enhance the overall success of the IVF process. Exercise helps regulate hormones, improve blood circulation, and promote both physical and mental well-being.
For women over 40 years of age, lifestyle changes like eating a nutrient-rich diet, maintaining a healthy weight, and avoiding toxins are crucial. Additionally, consult a fertility specialist for advanced options if natural methods alone don’t yield results.
High-intensity workouts are generally not recommended during the IVF cycle. Strenuous activities can stress the body and potentially disrupt the hormonal balance necessary for IVF success. It’s better to engage in low-impact exercises like walking, stretching, or prenatal yoga.
Participating in sports or high-impact activities during IVF treatment is usually discouraged, as they can increase the risk of injury and interfere with the IVF process. Low-impact activities such as swimming, walking, or gentle yoga are safer options that support the body during treatment.
In addition to appropriate physical activity, lifestyle changes that may improve IVF success include:
- Eating a balanced, fertility-supportive diet
- Reducing stress through relaxation methods like meditation or mindfulness
- Ensuring adequate sleep and rest
- Avoiding or limiting alcohol, tobacco, and caffeine
- Managing any underlying health conditions that could affect fertility
Yes, a common myth is that high-intensity workouts boost IVF success rates, which isn’t true—strenuous workouts can actually hinder the process. Another myth is that exercise should be completely avoided during IVF. In reality, light to moderate low-impact activities can support your physical and emotional well-being during treatment.
Most patients experience cramping for 24-48 hours after the procedure. Some might notice mild discomfort lasting up to 5 days, though this varies among individuals. The intensity typically peaks within the first few hours and gradually diminishes.
Cramping neither indicates success nor failure of the IUI procedure. These sensations simply show the body’s natural response to the treatment. Some successful pregnancies involve cramping, while others do not.
Doctors recommend several effective methods for managing post-IUI discomfort:
- Using a heating pad
- Taking approved pain relievers
- Staying well-hydrated
- Getting adequate rest
- Practising gentle stretching
A fertility specialist diagnoses and treats reproductive health conditions, advises individuals and couples on fertility treatments, and assists in developing personalized strategies to increase the likelihood of pregnancy. Their expertise is critical in identifying fertility issues and guiding patients through effective treatment plans.
Physical activity levels and stress can influence the intensity of cramping. Light activity is generally beneficial, but overexertion might increase discomfort. Maintaining a balanced lifestyle with adequate rest helps manage symptoms effectively.
To find the best gynecologist in Hyderabad, review their qualifications, specializations, and patient testimonials. Seek referrals from trusted sources like family, friends, or your general physician. Clinics such as Ferty9 are known for their experienced gynecologists and comprehensive reproductive health services.
Fertility medications can intensify cramping sensations. Different drugs affect patients differently, with hormone-based treatments potentially causing more noticeable effects. These medication-related symptoms typically resolve as the body adjusts.
Severe pain requires immediate medical attention, especially when accompanied by fever, heavy bleeding, or intense abdominal pressure. Patients should contact their fertility doctors if the pain becomes severe or persists beyond a week.
Yes, consulting a fertility specialist for IVF is essential. They evaluate your reproductive health, recommend suitable treatment protocols, and monitor the entire process. Their expertise ensures personalized care and enhances the chances of a successful pregnancy.
Yes, gynecologists can address fertility concerns by performing initial assessments, diagnosing common reproductive issues, and offering basic treatments like ovulation induction. They may also suggest lifestyle changes to improve fertility. For more complex cases, they refer patients to fertility specialists.
Fertility specialists typically recommend hormone evaluations, ovarian reserve tests, and ultrasounds for women. For men, semen analysis is conducted to assess sperm quality and quantity. Depending on individual circumstances, additional tests like genetic screening or laparoscopy may also be advised.
An ideal IVF clinic should offer state-of-the-art laboratory facilities, experienced fertility experts, and a wide range of diagnostic and treatment services. Look for clinics that provide individualized care, emotional support, and a high success rate. Ferty9 is one such clinic known for its advanced technology and holistic support.
The cost of consulting a fertility specialist varies based on the clinic and the specialist’s experience. Additional fees may apply if diagnostic tests or treatment procedures are required during the consultation. It’s best to inquire with the clinic for detailed pricing.
Diabetes can disrupt hormone levels and impair reproductive health in both men and women. In men, it can lead to erectile dysfunction, reduced sperm quality, and lower testosterone levels. In women, it may cause irregular menstrual cycles, ovulation problems, and hormonal imbalances that affect conception. Proper diabetes management is essential to support healthy reproductive function.
Some diabetes medications may influence fertility. For example, certain oral antidiabetic drugs or insulin therapies might affect hormonal balance or reproductive function in men and women. It’s important to consult your healthcare provider to understand how your treatment plan may impact fertility and explore alternative options if necessary.
Gestational diabetes typically resolves after childbirth. However, women who experience it are at a higher risk of developing Type 2 diabetes later in life. If left unmanaged, Type 2 diabetes can impact long-term fertility by causing hormonal and metabolic disruptions. Maintaining a healthy lifestyle post-pregnancy can help reduce this risk.
Moderate exercises such as walking, swimming, cycling, or yoga can enhance insulin sensitivity, maintain healthy body weight, and improve hormonal balance. These benefits can support fertility in individuals with diabetes. Consistent physical activity, combined with a balanced diet, is key to managing diabetes and boosting reproductive health.
Chromosomal abnormalities, such as numerical or structural anomalies, can significantly impact fertility. In men, disorders like Klinefelter syndrome (XXY) can result in low testosterone levels and reduced sperm production. In women, conditions like Turner syndrome (X0) can lead to ovarian dysfunction or premature ovarian failure, affecting their ability to conceive.
Yes, several genetic disorders can negatively affect sperm production in men. Examples include Klinefelter syndrome, Y chromosome microdeletions, and cystic fibrosis. These conditions can impair the production, quality, or motility of sperm, contributing to male infertility.
Signs may include hormonal imbalances, irregular menstrual cycles, or reduced sperm count in men. Consulting a fertility expert can help identify the root cause.
Assisted reproductive technologies (ART), such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), are highly effective in addressing certain genetic causes of infertility. These techniques help bypass natural barriers to conception and can increase the chances of pregnancy, even in the presence of genetic issues.
Yes, treatments like IVF and hormone therapy can improve fertility outcomes for individuals affected by reproductive hazards.
Epigenetics involves changes in gene expression that do not alter the DNA sequence but can be influenced by lifestyle and environmental factors. Factors like stress, poor diet, and chemical exposure can cause epigenetic changes that impact sperm and egg development, potentially contributing to infertility in both men and women.
Autosomal recessive disorders are genetic conditions inherited when both partners carry and pass on a mutated gene on a non-sex chromosome. These disorders may affect fertility either directly or through the risk of passing on serious health conditions to offspring. Genetic screening can help assess the risk and guide reproductive decisions.
Industries like agriculture, healthcare, chemical manufacturing, and radiation-based occupations are particularly risky.
Radiation can damage both male and female reproductive cells, leading to infertility or an increased risk of birth defects. It’s crucial to follow protective measures if working in radiation-prone environments.
Yes, mumps can affect the testicles in a condition known as mumps orchitis. This complication occurs in approximately 20-30% of males who contract mumps after puberty. Mumps orchitis is an inflammation of one or both testicles caused by the mumps virus travelling through the bloodstream and infecting the testicular tissue.
Yes, mumps can be prevented through vaccination. The mumps vaccine is typically administered as part of the measles-mumps-rubella (MMR) vaccine, which protects against all three viral diseases.
Yes, there is a vaccine for mumps. The mumps vaccine is typically combined with the measles and rubella vaccines in the MMR (measles-mumps-rubella) vaccine. This combination vaccine is routinely administered to children and provides long-lasting protection against all three viral diseases.
The MMR vaccine effectively prevents mumps, measles, and rubella. According to the Centres for Disease Control and Prevention (CDC), two doses of the MMR vaccine are approximately 88% effective in preventing mumps. However, the effectiveness can vary and depends on factors such as the individual’s age, immune status, and the strain of the mumps virus circulating in the community.
Yes, many women with autoimmune diseases can conceive and have healthy pregnancies. Proper management and medical support are essential to ensure both maternal and fetal well-being throughout the pregnancy.
Some medications are safe to use during pregnancy, while others may need to be adjusted or discontinued. It is important to consult your doctor before making any changes to your medication regimen to ensure the safety of both you and your baby.
Improving your chances of a healthy pregnancy involves preconception counseling, effective management of your autoimmune condition, and consistent monitoring throughout your pregnancy. Staying in close communication with your healthcare team is crucial for timely adjustments and ongoing care.
Autoimmune diseases can impact fertility in some individuals due to immune system dysfunction affecting reproductive processes. However, many people with autoimmune conditions can still conceive with proper medical care and, if necessary, additional fertility treatments tailored to their condition.
The IUI procedure itself is generally not considered painful, although some women may feel mild discomfort or cramping during the insertion of the catheter. Any discomfort is typically brief and well-tolerated.
The number of IUI cycles recommended can vary based on factors such as age, fertility history, and the underlying cause of infertility. Generally, most fertility specialists suggest trying 3-6 IUI cycles before considering more advanced treatments like in vitro fertilisation (IVF) if pregnancy is not achieved.
IUI is considered a relatively low-risk procedure. However, as with any medical procedure, there are some potential risks to be aware of, including:
- Mild cramping or spotting after the procedure
- Infection (rare)
- Multiple pregnancy (if fertility medications are used to stimulate ovulation)
- Ovarian Hyperstimulation Syndrome (OHSS)
Before starting IUI treatment, your fertility specialist may recommend several diagnostic tests to evaluate your fertility and identify any potential issues. These tests may include:
- Semen analysis for the male partner
- Ovarian reserve testing (e.g., AMH levels, antral follicle count)
- Evaluation of the uterine cavity (e.g., hysterosalpingogram or saline infusion sonography)
- Screening for infectious diseases
These tests help determine the most appropriate course of treatment and increase the chances of success with IUI.
Yes, thalassemia affects both men’s and women’s fertility. Premature ovarian aging, unpredictable menstrual periods, and hormonal imbalances can make it more difficult for women to conceive. In men, thalassemia often leads to reduced testosterone levels and impaired sperm production, further complicating fertility and reproductive health.
Yes, IUI can be performed for individuals with a history of miscarriage. However, your fertility specialist may recommend additional testing or treatment to address any underlying issues that may have contributed to the previous miscarriage(s).
Genetic counseling is crucial for couples affected by thalassemia. It provides valuable insights into the chances of passing the condition to their children and explains available reproductive options. Additionally, it offers emotional support and helps couples make informed decisions regarding family planning.
Yes, thalassemia can be managed during pregnancy to reduce potential risks, but it requires specialized care and regular monitoring. A multidisciplinary approach involving hematologists and obstetricians ensures a safer pregnancy for both mother and baby.
Couples with thalassemia can prepare to start a family by undergoing genetic counseling to understand their carrier status and assess the risk of inheritance. Maintaining good overall health, controlling iron and anemia levels, attending regular checkups, and consulting with specialists during the planning and pregnancy phases are all essential steps.
Stillbirth and preterm birth are some common outcomes of delayed parenthood or when a woman’s fertility age has passed. Additional complications may occur, especially during first-time pregnancies. Moreover, older couples often experience increased anxiety during the pregnancy phase compared to younger couples.
Signs that age may be impacting your fertility include irregular menstrual cycles, difficulty conceiving, or unexplained infertility between the ages of 30 and 40. Since the probability of pregnancy declines with age, it’s advisable to consult a fertility specialist and undergo tests to evaluate your reproductive potential.
Older couples who face challenges conceiving naturally can explore various fertility treatments and assisted reproductive technologies (ART). Some common options include:
- Ovulation induction
- In vitro fertilization (IVF)
- Egg donation
- Sperm donation
- Intrauterine insemination (IUI)
- Preimplantation Genetic Testing (PGT)
- Surrogacy
During a fertility assessment, you will typically be asked about your medical history to identify any underlying health conditions. Additional procedures may include a physical examination, ovulation tracking, semen analysis, hormone testing, genetic testing, and evaluation of ovulation timing.
Yes, polycystic ovary syndrome (PCOS) can increase the risk of miscarriage. PCOS is associated with metabolic dysfunction, which may result in poor reproductive outcomes. Elevated insulin levels and hormonal imbalances in women with PCOS are believed to play a significant role in increasing the risk of early pregnancy loss.
Yes, Ferty9 offers tailored fertility solutions for couples struggling to conceive, including those facing age-related fertility challenges. With a personalized treatment plan, Ferty9 helps improve the chances of achieving a healthy pregnancy—even beyond the conventional childbearing age.
Polycystic ovary syndrome (PCOS) can negatively impact fetal development and infant growth. It has been linked to variations in birth weight, birth length, head circumference, and even neurodevelopmental outcomes in early childhood. Close medical monitoring and proper management of PCOS during pregnancy can help reduce potential complications.
Yes, PCOS can affect fertility treatments like in vitro fertilization (IVF). Women with PCOS may have a higher risk of ovarian hyperstimulation syndrome (OHSS) and may experience challenges related to egg quality. However, with personalized treatment plans and close clinical supervision, successful outcomes with IVF are achievable for women with PCOS.
Yes, preterm labor is more common in pregnancies with PCOS. The condition is associated with an increased risk of complications such as hormonal imbalances, gestational diabetes, and hypertension, all of which can contribute to early labor. Regular prenatal care and early interventions are crucial in managing these risks effectively.
జీవనశైలి మార్పులు చేసుకోవడం, అండం విడుదలయ్యేలా చేయడం, IUI (అంతర్గర్భాశయ వీర్యకణాల ద్వారా అండం విడుదల) మరియు IVF (ఇన్ విట్రో ఫెర్టిలైజేషన్) వంటి చికిత్సా ఎంపికలు, కారణం తెలియని వంధ్యత్వానికి అందుబాటులో ఉన్నాయి. ఈ పద్ధతులు సంతానోత్పత్తిని పెంచడానికి, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచడానికి ఉద్దేశించబడ్డాయి మరియు యువ జంటలలో విజయవంతమైన గర్భాలకు దారితీయవచ్చు. ప్రతి ఒక్కరి శరీరం, ఆరోగ్య పరిస్థితి వేరుగా ఉంటాయి కాబట్టి, చికిత్సను వారి ప్రత్యేక అవసరాలకు తగ్గట్టుగా చేయడం వల్ల మంచి ఫలితాలు వస్తాయి.
కారణం తెలియని వంధ్యత్వాన్ని గుర్తించడానికి నిపుణులైన వైద్యులతో మీ పరిస్థితి గురించి చర్చించండి మరియు వివరణాత్మక సంతానోత్పత్తి పరీక్షలు చేయించుకోవాలి. తక్కువ ఇన్వాసివ్ చికిత్సా ఎంపికలను మొదటి వరుస చికిత్సగా పరిగణించాలి మరియు IUI మరియు IVF ఎంపికను ప్రత్యేక వైద్యులు అంచనా వేస్తారు.
కారణం తెలియని వంధ్యత్వానికి IVF యొక్క విజయ రేట్లు అందరికీ ఒకేలా ఉండవు. ఆరోగ్యకరమైన అండాశయ నిల్వ ఉన్న ఇతర మహిళలకు IVF ఎంత విజయవంతమవుతుందో, దీనికి కూడా దాదాపు అంతే ఫలితాలు ఉండవచ్చు. అయితే, మీ వయస్సు మరియు మీ ఇద్దరి ఆరోగ్య పరిస్థితిని బట్టి ఈ విజయ రేట్లు మారుతూ ఉంటాయి. సాధారణంగా, ఒక ప్రయత్నానికి 40 నుండి 50 శాతం వరకు గర్భం వచ్చే అవకాశం ఉంటుంది. ఒకసారి కాకపోతే, మళ్లీ ప్రయత్నించడం ద్వారా మొత్తం విజయం సాధించే అవకాశం ఇంకా ఎక్కువగా ఉంటుంది.
కారణం తెలియని వంధ్యత్వానికి IVF కంటే IUI తక్కువ విజయ రేటును కలిగి ఉంటుంది. అయితే, కొన్నిసార్లు, ముఖ్యంగా యువ జంటలకు మరియు పెద్ద సమస్యలు లేనివారికి, అండం విడుదలయ్యేలా చేసే మందులతో కలిపి IUI చేస్తే మొదటి ప్రయత్నంలోనే గర్భం వచ్చే అవకాశం ఉంది. ఇది IVF కంటే తక్కువ ఖర్చుతో కూడుకున్నది మరియు శరీరంపై ఎక్కువ ప్రభావం చూపదు. కానీ దీని విజయం మీ వయస్సు మరియు ఆరోగ్య పరిస్థితిపై ఆధారపడి ఉంటుంది.
ద్వితీయ వంధ్యత్వం అంటే ఇదివరకు ఒకసారి గర్భం దాల్చిన తర్వాత, వయస్సు పెరగడం, ఆరోగ్య సమస్యలు రావడం లేదా మగవారిలో సమస్యలు తలెత్తడం వంటి కారణాల వల్ల మళ్లీ గర్భం దాల్చడంలో ఇబ్బంది కలగడం. అయితే, కారణం తెలియని వంధ్యత్వం అంటే ఒక జంట కనీసం ఒక సంవత్సరం పాటు పిల్లల కోసం ప్రయత్నించినా, అన్ని సంతానోత్పత్తి పరీక్షలు సాధారణంగా వచ్చినప్పటికీ ఎందుకు గర్భం దాల్చలేకపోతున్నారో ఖచ్చితమైన కారణం తెలియకపోవడం.
Yes, XYY syndrome can be detected before birth through prenatal testing methods such as amniocentesis or chorionic villus sampling (CVS), which analyze the chromosomes of the developing baby.
Males with XYY syndrome are often taller than average. Increased height is one of the most consistent physical characteristics associated with the condition.
Most males with XYY syndrome have normal fertility and can father children naturally. However, a small percentage may experience reduced sperm counts, which could affect fertility.
Parents can support their child by creating a nurturing and structured environment, working with educators for personalized learning plans, and consulting healthcare professionals to address any medical or behavioral concerns early on.
Individuals with XYY syndrome may have a higher risk of developing anxiety, depression, or behavioral disorders. Early intervention, therapy, and emotional support can be effective in managing these challenges and promoting well-being.
The cost of prenatal DNA testing varies by location and the specific tests recommended by a genetic counselor. In India, it typically ranges from INR 20,000 to 30,000 but can be higher depending on the tests required.
You must consult a genetic counselor to choose the best prenatal genetic analysis for you. After evaluating the screening results, the counselor also provides suitable treatment options. Remember, different genetic disorders need specific treatments depending on the condition caused in the fetus.
Prenatal genetic testing can be carried out from the 10th week of pregnancy. It generally takes a week for the test results to arrive. It is, in fact, better to go through genetic testing as early as possible so that the genetic disorders are not detected at an advanced stage of your pregnancy.
The accuracy of genetic screening depends on the type of screening carried out, the kind of mutation being studied, and whether the condition has been previously identified in your family. While screenings like amniocentesis have a success rate of ~98%, genetic screenings might not be 100% accurate all the time.
Genetic testing before or during pregnancy can help prevent genetic disorders by identifying risk factors before symptoms appear. It allows individuals and couples to make informed reproductive choices, such as undergoing carrier testing to assess the risk of passing on disorders to their children. For couples using IVF, preimplantation genetic testing (PGT) can ensure only embryos without genetic abnormalities are implanted. Moreover, knowing one’s genetic risk can guide lifestyle and healthcare decisions to manage or mitigate potential health issues related to genetic predispositions.
No, genetic testing cannot predict or identify all potential fertility issues. Genetic testing can provide valuable insights into genetic factors that may contribute to infertility or increase the risk of passing on inherited conditions; many other factors can impact fertility, such as hormonal imbalances, structural abnormalities, environmental factors, and lifestyle choices. Genetic testing should be considered one component of a comprehensive fertility evaluation and treatment plan.
While PGT is generally considered a safe procedure, there are some potential risks to be aware of:
- Embryo damage: There is a small risk of damaging or losing embryos during the PGT process.
- Incorrect results: In rare cases, PGT results may give inaccurate values due to technical or human errors.
If a genetic compatibility test reveals that a couple has an increased risk of having a child with a genetic disorder, there are several options to consider:
- Preimplantation Genetic Testing (PGT): If the couple is pursuing IVF, PGT can help screen embryos and select those without the genetic condition of concern.
- Donor Gametes: The couple may consider using donor eggs or sperm to reduce the risk of passing on the genetic disorder.
- Adoption
- Acceptance of Risk: In some cases, couples may choose to accept the risk and go with natural conception or IVF without PGT.
The costs associated with genetic testing before IVF can vary and depend on the type of testing performed. It can range from the cost of basic genetic screening tests to more comprehensive tests like PGT, which may add additional costs for specialized care.
Yes, there are some risks associated with egg freezing. One of the primary risks is ovarian hyperstimulation syndrome (OHSS), which can result from the hormone stimulation process used to produce multiple eggs. Other potential issues may include side effects from medications, complications from the egg retrieval procedure, and emotional stress. It’s important to discuss these risks with a fertility specialist before proceeding.
Corporate support has significantly influenced the growing trend of egg freezing by making the procedure more affordable and accessible. Many companies now offer fertility benefits, including egg freezing coverage and assistance programs. This support empowers women to explore fertility preservation options without immediate financial strain, allowing them to focus on career development while planning for future family building.
Education plays a crucial role in the decision to freeze eggs. Awareness about reproductive health, fertility timelines, and technological advancements enables women to make informed decisions. Understanding the benefits, limitations, and procedures of egg freezing helps women align their fertility choices with both their career and personal life goals, promoting proactive reproductive planning.
In some cases, cryopreservation may lead to embryonic damage, resulting in DNA fragmentation and reduced developmental potential. While the process is generally safe and widely used, it is important to understand that, like any medical procedure, it carries certain risks and limitations. Consultation with a fertility expert can help evaluate the benefits and potential downsides based on your individual case.
Ferty9’s Onco Freezing service helps cancer patients by preserving their fertility before undergoing treatments. Oocytes (eggs), sperm, or embryos are safely collected and frozen. After completing cancer therapy or other critical medical treatments, patients can use these preserved reproductive materials to start or grow their families, offering hope and planning beyond their health journey.
Success rates with cryopreserved embryos or eggs in IVF are generally high. Advances in freezing techniques, such as vitrification, have improved survival rates after thawing and increased the likelihood of successful implantation and pregnancy. Many couples benefit from this option when planning delayed parenthood or undergoing fertility treatment.
Studies have shown that using cryopreserved embryos, including in procedures like intracytoplasmic sperm injection (ICSI), does not increase the risk of birth defects or developmental issues. The outcomes are consistent and reassuring, making cryopreservation a reliable option for preserving reproductive potential without additional genetic risks.
Excessive exercise can negatively affect fertility. Overexertion may lead to hormonal imbalances, a decrease in body fat, and an energy deficiency. These factors can disrupt the reproductive system and impact fertility if sustained over time.
Yes, starting a new low- to moderate-intensity exercise routine can be safe and beneficial when trying to conceive. It helps improve physical health, which is important for pregnancy and childbirth.
Exercise can support hormonal regulation, especially hormones associated with ovulation and the menstrual cycle. By promoting hormonal balance, exercise may enhance fertility and improve the success rate of IVF treatments.
Low to moderate-intensity exercise can help reduce stress, regulate hormones, manage weight, and improve insulin sensitivity. These benefits support regular menstrual cycles and ovulation. However, excessive exercise may negatively impact menstrual health.
In women with a normal body weight, vigorous exercise may reduce fertility. However, moderate physical activity generally improves fertility in all women. Excessive exercise can also lower the success rates of fertility treatments like IVF.
To improve fertility rates naturally, consider adopting a healthy lifestyle by maintaining a balanced diet regimen, exercising regularly, managing stress, and avoiding harmful substances like tobacco and excessive alcohol consumption. Additionally, tracking ovulation cycles and timing intercourse accordingly can increase the chances of conception.
Fertility rates play a significant role in family planning decisions. Couples may choose to have fewer children in areas with declining fertility rates or delay childbearing due to concerns about age-related fertility decline. Conversely, in regions with high fertility rates, access to family planning resources and education becomes crucial for managing population growth and promoting reproductive health.
Modern medical advancements, such as assisted reproductive technologies, have significantly impacted male and female fertility. Techniques like in vitro fertilisation (IVF), intracytoplasmic sperm injection (ICSI), and preimplantation genetic testing have helped couples overcome various infertility challenges. Additionally, advancements in hormone therapies, surgical procedures, and diagnostic tools have improved the understanding and treatment of infertility in both genders.
Yes, uterine tuberculosis (TB) can be asymptomatic, especially in the early stages. Many women with uterine tuberculosis may not exhibit visible symptoms, or their symptoms are mild, making the condition difficult to diagnose without thorough medical testing.
Uterine tuberculosis (TB) is not directly communicable. However, uterine tuberculosis occurs when tuberculosis bacteria from other parts of the body, most notably the lungs, move to the reproductive organs via the bloodstream or lymphatic system. This indicates that uterine tuberculosis is caused by bacteria spreading internally rather than through sexual or close physical contact with others.
Treatment for uterine tuberculosis (TB) usually entails a long course of anti-tuberculosis medications that can last anywhere from a few months to a year, depending on the severity of the illness and the individual response to treatment.
Yes, in vitro fertilization (IVF) can help women with infertility caused by uterine tuberculosis (TB), especially when the damage to the reproductive organs—especially fallopian tubes—is extensive and cannot be easily addressed with medicine or surgery.
Yes, foods high in sugar, caffeine, and unhealthy fats can increase inflammation and worsen cramps. Avoid processed and fried foods for better period pain relief.
The effects vary from person to person. Consistently eating a diet rich in anti-inflammatory foods may reduce cramps in a few cycles.
Foods like papaya, pineapple, and ginger may stimulate uterine contractions and slightly advance your period. However, consult a healthcare provider before trying this approach.
Some of the most common toxic chemicals that can negatively impact female egg counts include persistent organic pollutants (POPs), phthalates, heavy metals like lead and mercury, and bisphenol A (BPA).
Yes, reducing exposure to toxic chemicals can improve your egg count or slow its decline. You can also protect your ovarian reserve and maintain a healthy egg supply.
Early signs that toxic chemicals may be affecting your fertility include irregular menstrual cycles, hormonal imbalances, and difficulty conceiving.
While there is limited scientific evidence on the effectiveness of detox diets or supplements, some experts suggest that a balanced diet rich in antioxidants and certain supplements, such as N-acetyl-cysteine (NAC), may help support the body’s natural detoxification processes and potentially mitigate the effects of toxic chemicals on egg count.
Exposure to toxic chemicals during pregnancy can have significant impacts on the developing fetus. These chemicals can move across the placental barrier and potentially lead to congenital disabilities, developmental delays, and an increased risk of childhood health issues like learning disabilities, behavioural problems, and certain types of cancer.
BBT monitoring cannot accurately predict ovulation during IVF treatment because the medications used alter natural temperature patterns. Doctors rely on blood tests and ultrasound monitoring to track follicular development and determine the optimal timing for egg retrieval.
Temperature tracking after embryo transfer is not recommended as it may cause unnecessary stress. The medications used during this phase will affect temperature patterns, making readings unreliable indicators of treatment success.
IVF medications significantly impact temperature patterns in several ways:
- Stimulation drugs can elevate baseline temperatures
- Progesterone supplements cause sustained temperature increases
- Trigger shots create temporary temperature spikes
- Support medications may mask natural patterns
While BBT tracking is not essential during IVF, some patients find it helpful for understanding their body response to treatment. Doctors focus primarily on other monitoring methods, such as blood tests and ultrasounds, for treatment decisions.
For some patients, temperature tracking provides a sense of control and involvement in their treatment. However, others may find it adds unnecessary anxiety. Doctors recommend discussing individual preferences with the healthcare team to determine if BBT monitoring would be beneficial.
Research indicates that effective stress management can significantly improve fertility outcomes in men. Studies show that men who successfully reduce their stress levels often experience improvements in sperm quality, including better motility and concentration. These improvements can be attributed to the normalization of hormone levels, particularly testosterone, which plays a crucial role in sperm production.
Employers can create fertility-supportive environments through comprehensive workplace policies. Key support measures include:
- Implementing flexible working arrangements
- Providing access to fertility testing and treatments
- Offering counselling services
- Training leadership in fertility awareness
- Creating Inclusive Health Policies
- Establishing stress management programmes
Most men begin to notice improvements in their overall well-being within 4-8 weeks of implementing stress management techniques. However, since sperm production takes approximately three months, meaningful changes in fertility parameters typically become evident after this period. Consistently practising stress reduction techniques is essential for maintaining these improvements.
Chronic workplace stress can lead to several long-term reproductive health challenges:
- Permanently altered hormone production
- Reduced sperm quality and quantity
- Decreased testosterone levels
- Impaired testicular function
- Compromised reproductive capacity
Managing stress is crucial during the IVF process, as elevated levels of stress can disrupt the delicate hormonal balance required for ovulation and embryo implantation, potentially reducing the chances of a successful pregnancy. Additionally, stress can take a toll on an individual’s emotional and physical well-being, making it more challenging to cope with the demands of fertility treatments.
Some common signs that you might benefit from IVF counselling include:
- Experiencing overwhelming feelings of anxiety, sadness, or frustration
- Struggling to cope with the emotional ups and downs of the IVF process
- Feeling isolated or unsupported
- Experiencing relationship strain or communication issues with your partner
- Difficulty managing stress or developing healthy coping mechanisms
Absolutely. Counselling can provide a safe and supportive ecosystem where one can express their feelings and concerns without fear of judgment. A skilled counsellor can help you develop strategies for building a support network and fostering connections with others who understand the unique challenges of the IVF journey.
While fertility specialists are primarily responsible for the medical aspects of IVF treatment, they can also play a crucial role in managing stress. Many fertility clinics have counsellors on staff or can refer to mental health professionals who specialise in fertility-related issues. Your fertility specialist can also offer guidance on stress management methods and resources to support your emotional well-being throughout the process.
While there is no definitive age at which sperm count begins to decline, research suggests that a gradual decrease in sperm production can start as early as the late 30s or early 40s for some men. However, the rate and extent of this decline can vary significantly among individuals.
To maintain healthy sperm as they age, men can adopt a balanced diet rich in nutrients like antioxidants, zinc, and vitamin C, engage in regular physical exercise, manage stress effectively, avoid harmful habits like smoking, illicit drug usage, excessive alcohol consumption, and schedule regular health checkups to monitor their reproductive health.
The most noticeable effects of ageing on sperm quality include decreased sperm count, reduced sperm motility (the ability to swim effectively), abnormal sperm morphology (shape and structure), and an increased risk of sperm DNA fragmentation.
Yes, lifestyle alterations can play a significant role in mitigating age-related sperm decline. Adopting a healthy, well-balanced diet, avoiding junk food, engaging in regular exercise, maintaining a healthy body weight, managing stress, and avoiding illicit habits like smoking and excessive alcohol drinking can all contribute to preserving sperm health as men age.
To monitor your sperm health as you age, doctors generally recommend scheduling regular semen analyses or sperm count tests. These tests can provide valuable insights into sperm count, motility, morphology, and overall reproductive health. Additionally, discussing any concerns or changes with your doctor is essential for early detection and appropriate management of potential issues.
According to the World Health Organisation (WHO), a normal range for sperm morphology is considered to be 4% or higher. However, it’s important to note that this is a general guideline, and individual fertility specialists may have different criteria based on their experience and clinical practices.
Yes, there are various types of sperm abnormalities that can be observed during a morphology assessment. These include head defects (such as abnormal shape, size, or the presence of vacuoles), midpiece defects (abnormalities in the structure or size of the midpiece), and tail defects (abnormalities in the length, shape, or presence of the tail).
Lifestyle changes can improve sperm morphology in several ways:
- Maintaining a healthy diet routine: A balanced diet rich in antioxidants, minerals, and vitamins can protect sperm cells from oxidative damage and support their proper development and structure.
- Regular physical activity: Moderate exercise can improve blood flow, reduce oxidative stress, and maintain a healthy weight, all of which can contribute to better sperm morphology.
- Avoiding harmful substances: Quitting smoking, limiting alcohol, and avoiding recreational drugs can prevent damage to sperm cells and improve their overall quality, including morphology.
- Managing stress and sleep: Chronic stress and lack of sleep can disturb hormonal balance and negatively impact sperm production and quality. Practising stress-management techniques and getting adequate sleep can support healthy sperm morphology.
Yes, age can have an impact on sperm morphology. As men get older, the quality of their sperm, including morphology, tends to decline. This is due to various factors, such as decreased testosterone levels, oxidative stress, and the accumulation of genetic mutations over time.
While regular moderate exercise is generally recommended to support overall sperm health, no specific exercises have been proven to improve sperm morphology directly. However, exercises that promote blood flow and reduce oxidative stress, such as aerobic activities (e.g., brisk walking, jogging, swimming) and strength training, may positively impact sperm quality, including morphology.
Doctors use multiple screening methods throughout pregnancy to monitor pre-eclampsia risk. The primary screening tools include regular blood pressure monitoring, urine protein tests, and blood tests that measure specific biomarkers. Modern clinics also utilise pre-eclampsia risk calculators considering various factors, including medical history and current symptoms.
PICSI (Physiological Intracytoplasmic Sperm Injection) represents an advanced sperm selection method that helps identify sperm with optimal DNA quality. This technique mimics natural selection processes, allowing embryologists to choose sperm cells with better genetic integrity, potentially reducing the risk of pregnancy complications.
Fertility specialists recommend conducting a sperm DNA fragmentation test before starting IVF treatment and monitoring changes every three months if initial results show concerns. This timeline aligns with the natural sperm production cycle and allows for the assessment of improvement strategies.
Preparation should begin at least three months before IVF treatment. Key steps include:
- Comprehensive health screening for both partners
- Implementation of lifestyle modifications
- Regular monitoring of sperm DNA quality
- Early consultation with specialists about pre-eclampsia prevention strategies
Yes, specific lifestyle modifications can significantly impact sperm DNA quality. Research shows that achieving a healthy weight, reducing exposure to environmental toxins, and following a balanced diet rich in antioxidants can help improve DNA fragmentation index scores within three to six months.
No, PCOD is not related to sexual activity. PCOD is a condition characterised by the presence of multiple small fluid-filled sacs or cysts on the ovaries, and it can occur in both sexually active and non-sexually active women.
Both PCOD and PCOS have a genetic component, meaning they can run in families. However, environmental factors, like diet, lifestyle, and weight, also play a significant role in the development and progression of these conditions.
If you have been diagnosed with PCOD or PCOS, it is recommended to have regular follow-up appointments with your doctor, typically every 6 to 12 months. These follow-ups enable your doctor to monitor your condition, adjust treatment plans (if necessary), and screen for potential long-term health risks.
Yes, PCOD can occur or persist after marriage. PCOD is a condition related to the ovaries and hormonal imbalances, and it is not directly affected by marital status. Women with PCOD may experience symptoms and potential fertility issues before or after getting married.
Women with PCOS often experience a range of challenges, including:
Irregular Menstrual Cycles: PCOS can cause irregular or missed periods, making it difficult to predict ovulation and plan for pregnancy.
Difficulty Conceiving: Hormonal imbalances can make it challenging to conceive naturally. Many women with PCOS may require fertility treatments.
Insulin Resistance and Metabolic Issues: PCOS is linked to insulin resistance, which can lead to weight gain, type 2 diabetes, and other metabolic problems.
Hirsutism and Acne: Elevated androgen levels can cause unwanted hair growth and acne.
Emotional and Mental Health Challenges: The physical symptoms and emotional toll of PCOS can contribute to anxiety, depression, and body image concerns.
You should consider consulting a reproductive health specialist if you experience any of the following signs:
– Irregular or absent menstrual periods
– Excessive hair growth (hirsutism)
– Severe acne or oily skin
– Difficulty conceiving or infertility
– Unexplained weight gain or obesity
– Pelvic pain or discomfort
– Hormonal imbalances or noticeable changes
A reproductive health specialist can offer a thorough evaluation, order diagnostic tests, and recommend a personalised treatment plan based on your condition.
Maintaining good reproductive health involves a mix of healthy lifestyle habits and regular medical care. Key practices include:
Balanced Diet and Exercise: Eat nutrient-rich foods and stay physically active to manage weight and hormonal health.
Stress Management: Use stress-relief techniques like yoga, meditation, or therapy to support hormonal balance.
Avoid Harmful Substances: Avoid smoking, limit alcohol intake, and reduce exposure to environmental toxins.
Regular STI Screenings: Early detection and treatment of STIs are crucial to preventing reproductive issues.
Annual Check-ups: Schedule well-woman exams, pap smears, and screenings relevant to your age and risk profile.
Open Communication with Doctors: Be honest about your symptoms and medical history to ensure accurate diagnosis and care.
Seed cycling may help regulate irregular periods by supporting hormonal balance. The nutrients found in seeds can contribute to regulating oestrogen and progesterone levels, which play a crucial role in the menstrual cycle.
The time it takes to see results from seed cycling can vary from individual to individual. Some women may experience improvements in menstrual regularity or alleviation of symptoms within a few cycles, while others may require several months of consistent seed cycling. Its essential to be patient and consistent with the practice, as hormonal balance can take time to achieve.
This process is generally considered safe when followed correctly and with the appropriate seed quantities. However, some women may experience mild digestive discomfort, such as bloating or gas, due to the increased fibre intake from seeds. Its recommended to gradually increase your seed intake and stay well-hydrated to minimise these potential side effects.
While seed cycling primarily supports hormonal balance during the reproductive years, some women may find it beneficial during the perimenopausal and early menopausal stages. The nutrients found in seeds, such as phytoestrogens and essential fatty acids, may help alleviate certain menopause symptoms like mood changes, hot flashes, and vaginal dryness. However, more research is needed in this area, and its advisable to consult with a doctor for personalized guidance during this transition.
Yes, there are a few fertility risks, such as miscarriage, preeclampsia, preterm birth, cesarean delivery, gestational diabetes, and pregnancy-induced high blood pressure associated with PCOS. It is essential to work closely with a fertility specialist to understand and manage these risks effectively.
Women with PCOS should manage stress carefully, as it can cause hormonal imbalances that affect ovulation and fertility. Stress may alter levels of estrogen, progesterone, and thyroid hormones, which are directly associated with the reproductive system. Mindfulness, therapy, and lifestyle changes can help in reducing stress and improving fertility outcomes.
It is possible to conceive with PCOS without medical intervention, but it may take longer. Women with PCOS often face issues such as hormonal imbalances, weight challenges, and irregular ovulation. While some may conceive naturally, many benefit from medical support to improve their chances of a successful pregnancy.
Yes, natural remedies such as taking vitamin D supplements, engaging in regular physical activity, avoiding excessive caffeine, ensuring adequate sleep, and eating a balanced diet can help regulate menstrual cycles in women with PCOS. These lifestyle adjustments may support hormonal balance and improve reproductive health.
Insulin resistance plays a key role in PCOS-related fertility issues. It affects the production and regulation of hormones involved in the menstrual cycle. Elevated insulin levels can disrupt ovulation and contribute to hormonal imbalances, making it more difficult for women with PCOS to conceive. Managing insulin levels through diet, exercise, and medication can improve fertility outcomes.
Yes, PCOD is a primary cause of irregular periods as it is a hormonal disorder in which the ovaries tend to produce excess androgens (male hormones). Elevated male hormone (androgen) levels may disrupt egg maturation and release, leading to irregular periods. Ovaries may develop cysts containing immature eggs, contributing to irregular menstruation.
The symptoms of PCOD can show its effect in various ways, that includes: irregular periods, absence of periods, heavy menstrual bleeding, and painful periods. Periods that are less frequent have less than eight per year, with cycles longer than 35 days. Absence of periods lasts for three months or longer, and irregular periods vary significantly in duration, making it difficult to predict when the next period will occur.
Maintaining a balanced weight is very important for women with PCOD, as insulin resistance can lead to increased androgen production which leads to irregularities in periods. Excess weight, especially abdominal fat, can worsen these issues. Weight loss can improve insulin sensitivity and lower the level of androgens. As a result, PCOD-related complications will also be reduced.
Natural remedies for menstrual irregular periods (PCOD) include a low Glycaemic Index (GI) diet, anti-inflammatory diet, limiting processed foods, herbal supplements, stress management techniques, and enough sleep. These remedies will support overall health and improve PCOD symptoms, but should be discussed with a healthcare provider. Regular exercise improves insulin sensitivity, weight management, and hormonal balance.
Medical treatments for menstrual cycle regulation in female partners may include hormonal control, progestin therapy, diabetic controlling medicines, anti-androgen medicines, and ovulation-promoting medicines. The suitable treatments advised by fertility experts will help to manage symptoms, reduce any long-term complications, and promote regular ovulation and improve chances for pregnancy.
Lifestyle changes are crucial for managing PCOD and improving period regularity. A balanced diet low in processed and refined sugars, rich in fibre and whole foods, stabilizes blood sugar levels, improves insulin sensitivity, and supports hormonal balance. Regular exercise, weight management, and maintaining a healthy weight are essential factors. Stress management and adequate sleep are crucial for hormonal health, regulating cortisol levels and affecting other hormones involved in the menstrual cycle.
Pregnancy with PCOS and irregular periods can be challenging due to disrupted ovulation cycles. To avoid irregular periods, follow a healthy lifestyle, including a healthy diet, regular exercise, and stress reduction. If lifestyle changes aren’t enough, medical intervention suggested by fertility experts, timed intercourse, Intrauterine Insemination (IUI), and In Vitro Fertilization (IVF) can be used. These treatments can help overcome ovulation and fertilization challenges.
Yes, implantation bleeding is considered one of the earliest signs of pregnancy and can occur even after a successful implantation. However, it’s important to note that not all females experience implantation bleeding.
It is possible to experience implantation bleeding around the time of a missed period. Implantation bleeding typically occurs seven to twelve days after conception, which coincides with the expected timing of a menstrual period. If you experience light bleeding or spotting around the time of your expected period and have missed your period, it could be an indication of implantation bleeding and potential pregnancy.
No, implantation bleeding does not always occur with pregnancy. Some women may experience implantation bleeding, while others may not. The absence of implantation bleeding does not necessarily mean that pregnancy has not happened.
While implantation bleeding is generally considered normal, there are certain signs that may indicate an abnormal or concerning situation:
Heavy bleeding: Implantation bleeding is typically light and resembles spotting or a brown discharge. If the bleeding becomes heavy or resembles a standard period, it could be a sign of a problem.
Severe cramping or pain: Mild cramping may occur during implantation bleeding, but severe cramping or pain could indicate potential hazards such as an ectopic pregnancy.
Prolonged bleeding: Implantation bleeding typically lasts for 1–3 days. If the bleeding persists for more than a few days, it may cause concern.
Clotting: Significant clotting is not common with implantation bleeding and could be a sign of an issue.
Yes, vaginal swelling is a common symptom during pregnancy due to hormonal changes, increased blood flow, and fluid retention.
For most women, swollen labia in pregnancy resolves on its own a few weeks after delivery. However, if the swelling persists, consult your doctor.
While a swollen vulva during pregnancy is usually harmless, you should seek medical attention if it’s accompanied by severe pain, unusual discharge, or fever, as this could indicate an infection or other complications.
Simple measures like using cold compresses, taking sitz baths, wearing loose clothing, and staying hydrated can relieve swelling Labia While Pregnant. If these don’t work, consult your doctor for further evaluation. By understanding and addressing labia swelling in pregnancy, you can enjoy a healthier and more comfortable pregnancy experience.
Fertility yoga retreats typically offer practices and guidance tailored to support both male and female fertility. While certain poses and techniques may be more focused on supporting ovarian function or hormonal balance for women, there are also practices designed to improve sperm health and overall reproductive wellness for men. The stress-reducing and mindfulness aspects of fertility yoga can also benefit individuals of all genders on their fertility journey.
Many fertility yoga retreats incorporate dietary recommendations or meal plans to support reproductive health and overall well-being. These may include an emphasis on nutrient-dense, whole foods and the inclusion of fertility-friendly ingredients like leafy greens, healthy fats, and antioxidant-rich fruits and vegetables. Certain yoga retreats may also guide you on reducing exposure to potential endocrine disruptors or inflammatory foods.
Along with the physical benefits, fertility yoga retreats often prioritize emotional and mental well-being, which can be particularly important for those navigating the challenges and stress associated with fertility struggles. Through various practices, like meditation, breathwork, and mindfulness exercises, retreats can help participants cultivate a sense of calm, reduce anxiety, and develop strategies to manage the emotional fluctuations of their fertility journey.
Yes, fertility yoga retreats can be a valuable complement for individuals undergoing fertility treatments like in vitro fertilization (IVF) or intrauterine insemination (IUI). The practices taught at these retreats can help manage stress and improve overall well-being – factors that may positively impact the success rates of fertility treatments. However, it’s essential to consult with your doctor and ensure that any physical practices are tailored to your specific treatment protocol and stage of the process.
అవును, గర్భధారణ సమయంలో యోని వాపు రావడం సాధారణమైన విషయమే. హార్మోన్లలో మార్పులు, రక్త ప్రసరణ పెరగడం మరియు శరీరంలో నీరు నిలుపుకోవడం వల్ల ఇలా జరుగుతుంది.
చాలా మంది మహిళలకు, గర్భధారణ సమయంలో వచ్చిన యోని వాపు డెలివరీ అయిన కొన్ని వారాల తర్వాత వాటంతట అదే తగ్గిపోతుంది. ఒకవేళ వాపు తగ్గకపోతే, మీ డాక్టర్ను సంప్రదించండి.
గర్భంతో ఉన్నప్పుడు యోని ఉబ్బడం సాధారణంగా ప్రమాదకరం కాదు. కానీ, మీకు చాలా నొప్పిగా ఉంటే, దుర్వాసన తో కూడిన తెల్లబట్ట అవుతుంటే లేదా జ్వరం వస్తే మాత్రం డాక్టర్ను తప్పకుండా కలవాలి. అలా జరిగితే ఇన్ఫెక్షన్ లేదా ఇతర సమస్యలు ఉండొచ్చు.
చల్లటి నీటితో తడపడం, గోరువెచ్చని నీటిలో కూర్చోవడం (సిట్జ్ బాత్), వదులుగా ఉండే బట్టలు వేసుకోవడం, మరియు బాగా నీరు తాగడం వంటి చిన్న చిన్న పనులు చేయడం వల్ల వాపు తగ్గుతుంది. ఒకవేళ తగ్గకపోతే, డాక్టర్ను కలిసి పరీక్ష చేయించుకోండి. గర్భధారణలో యోని పెదవుల వాపు గురించి తెలుసుకోవడం మరియు దానికి తగినట్లుగా చేసుకోవడం ద్వారా, మీరు ఆరోగ్యకరమైన మరియు సౌకర్యవంతమైన గర్భధారణను ఆస్వాదించవచ్చు.
Yes, it’s completely normal. Most healthy couples conceive within 6 to 12 months of trying. If you’re under 35 and have only been trying for three months, there’s usually no cause for concern. Doctors typically recommend waiting up to a year before pursuing fertility evaluations.
You should consider seeing a fertility specialist if you’re under 35 and haven’t conceived after 12 months of regular, unprotected intercourse. If you’re over 35, seek help after six months of trying. Immediate evaluation is also recommended if you have known reproductive issues or irregular menstrual cycles.
Yes, high stress levels can impact your ability to conceive. Stress can disrupt hormone balance, interfere with ovulation in women, and reduce sperm quality in men. Managing stress through relaxation techniques, exercise, and emotional support can help improve fertility.
You can detect ovulation using several methods, including tracking basal body temperature, using ovulation predictor kits (OPKs), observing changes in cervical mucus, or using fertility tracking apps. These tools can help you identify your most fertile days for conception.
Healthy lifestyle habits can enhance fertility. These include eating a balanced diet, maintaining a healthy weight, exercising regularly, limiting alcohol and caffeine intake, quitting smoking, and managing stress effectively. These changes can support hormone regulation and reproductive health.
ఈస్ట్ ఇన్ఫెక్షన్లు మహిళల్లో చాలా సాధారణం అయినప్పటికీ, పురుషులకు కూడా రావచ్చు. వీర్యంలో ఈస్ట్ ఇన్ఫెక్షన్లు ఎంత సాధారణమో ఖచ్చితంగా తెలియదు, కానీ ఇతర జననేంద్రియ ఇన్ఫెక్షన్లతో పోలిస్తే ఇవి చాలా అరుదుగా వస్తాయి.
వైద్యులు శారీరక పరీక్ష మరియు ల్యాబ్ పరీక్షల ద్వారా వీర్యంలో ఈస్ట్ ఇన్ఫెక్షన్ను నిర్ధారించగలరు. ఇందులో ఈస్ట్ ఇన్ఫెక్షన్ నుండి స్రావం లేదా ప్రభావిత చర్మం యొక్క నమూనాను సేకరించి మైక్రోస్కోప్లో చూడటం లేదా ఏ రకమైన ఈస్ట్ ఉందో గుర్తించడానికి కల్చర్ చేయడం వంటివి ఉండవచ్చు.
ప్రోబయోటిక్స్ వంటి కొన్ని సహజ నివారణలు శరీరంలో సూక్ష్మజీవుల సరైన సమతుల్యతను పునరుద్ధరించడంలో సహాయపడవచ్చు, అయితే సహజ నివారణలతో మాత్రమే ఈస్ట్ ఇన్ఫెక్షన్కు చికిత్స చేయడానికి ప్రయత్నించే ముందు వైద్యుడిని సంప్రదించడం చాలా ముఖ్యం. కొన్ని సందర్భాల్లో, మీ డాక్టర్ సూచించిన ఈస్ట్ ఇన్ఫెక్షన్ మందులు అవసరం కావచ్చు.
ఒక భాగస్వామికి ఈస్ట్ ఇన్ఫెక్షన్ ఉంటే, ఇన్ఫెక్షన్ ఒకరి నుండి మరొకరికి తిరిగి వ్యాప్తి చెందకుండా నిరోధించడానికి ఇద్దరు భాగస్వాములు ఈస్ట్ ఇన్ఫెక్షన్ చికిత్స తీసుకోవాలని వైద్యులు సిఫార్సు చేస్తున్నారు. మీ డాక్టర్ సరైన చికిత్స ప్రణాళికపై మీకు మరియు మీ భాగస్వామికి మార్గనిర్దేశం చేయగలరు.
క్రీమ్లు లేదా సపోజిటరీల వంటి ఓవర్-ది-కౌంటర్ యాంటీ ఫంగల్ చికిత్సలు వీర్యంలోని తేలికపాటి ఈస్ట్ ఇన్ఫెక్షన్లకు సమర్థవంతంగా చికిత్స చేయగలవు. అయితే, సూచనలను పాటించడం మరియు లక్షణాలు కొనసాగితే లేదా తీవ్రతరం అయితే వైద్యుడి సలహా తీసుకోవడం చాలా ముఖ్యం. కొన్ని సందర్భాల్లో, యాంటీ ఫంగల్ మందులు అవసరం కావచ్చు.
Yes, stress can interfere with hormonal balance, making it harder for couples trying to conceive. High cortisol levels can disrupt ovulation in women and reduce sperm quality in men.
You can reduce stress by practicing mindfulness, yoga, regular exercise, and seeking emotional support. Professional counseling and relaxation techniques also help manage stress when trying to conceive.
Yes, depression when trying to conceive can lower your chances by affecting hormonal balance and overall health. Addressing mental health is essential for improving fertility.
To manage stress, focus on healthy habits like a nutritious diet, adequate sleep, and staying active. Engaging in hobbies, spending quality time with loved ones, and seeking professional guidance can also ease the process of how to get pregnant.
Metrorrhagia refers to irregular or abnormal vaginal bleeding that is not a part of the normal menstrual cycle. On the other hand, menorrhagia specifically describes heavy or prolonged menstrual bleeding during the regular menstrual period.
Untreated metrorrhagia can lead to complications such as anaemia, fatigue, and an increased risk of developing infections or other health issues. Along with physical symptoms, irregular bleeding can cause emotional and psychological stress and disrupt daily activities. It is essential to seek medical attention to identify and address the underlying cause.
Doctors generally advise avoiding strenuous exercise during episodes of metrorrhagia, as it may exacerbate the bleeding. However, low-impact activities may be permitted, depending on the severity of the bleeding and your doctor’s advice.
The time required to diagnose metrorrhagia can vary and depends on the individual’s symptoms, medical history, and the complexity of the underlying cause. In some cases, a diagnosis can be made after an initial physical examination and basic tests, while in others, additional testing or evaluation may be necessary.
The symptoms of ovulation include changes in cervical mucus, breast tenderness, lower abdominal pain, light spotting or discharge, a change in basal body temperature, libido changes, headache, and sometimes nausea.
Start tracking your menstrual cycle on a calendar to see what’s typical for you. Begin by noting your start date every month for several months to determine the frequency of your periods. There are several online apps that can help you track your menstrual cycle.
Hormone fluctuations are one of the many elements of the body’s stress response. When your hormones become unbalanced for a long duration, hormone-driven systems such as reproduction are frequently affected.
Your menstrual cycle fluctuates significantly during your life for essential reasons. Puberty occurs in adolescence, followed by your most fertile years, which start in your late teens and end in your early 20s. Your menstrual cycle may shift from decade to decade, covering from teen to perimenopause and menopause.
Adequate protein, plant-based fats, and high-fiber carbs provide your body with the resources it needs to produce the right quantity of hormones. Drinking an adequate amount of water, iron-rich foods, Omega-3 fish oil, and certain herbs such as ginger and cinnamon helps relieve menstrual cramps, abdominal bloating, depression, and mood swings.
Consult your doctor if your periods are irregular. It is essential to investigate the cause and potential health risks of this, even if it doesn’t affect you. If your menstruation is late or has stopped, you should get a pregnancy test done.
The most common reasons behind false positive results include chemical pregnancies, medication interactions, and testing errors. Doctors identify these primary factors: recent fertility treatments, certain medications containing hCG, testing too soon after pregnancy loss, reading tests outside the time window, and expired test materials.
Yes, a late period can indicate pregnancy even with a negative test result. Hormone levels might be too low for detection, especially if testing occurs too early. Doctors recommend waiting 48–72 hours before retesting if pregnancy is suspected.
Most pregnancy tests are most accurate when taken the day after a missed period. However, some highly sensitive tests can detect pregnancy hormones earlier. Testing should occur during morning hours when hormone concentrations are highest for optimal accuracy.
Several medical conditions can affect pregnancy test results. These include certain ovarian cysts, pituitary disorders, and some rare tumours. Individuals with these conditions should consult doctors for proper evaluation and testing guidance.
Testing should occur 1–2 days after a missed period for maximum accuracy. Morning testing provides the most reliable results due to concentrated hormone levels. If uncertainty exists, doctors recommend waiting one week after a missed period for definitive results.
Genetics, maternal health, and proper nutrition all impact fetal development, which is essential for healthy growth. Lifestyle choices—such as avoiding smoking, alcohol, and drugs, and limiting exposure to pollutants—are crucial. Regular prenatal care allows for early detection and management of any issues.
Yes, stress can affect fetal health during pregnancy. High stress levels can increase the risk of preterm birth (before 37 weeks) and low birth weight. Prolonged stress may also contribute to complications like high blood pressure and heart disease.
Nutrition is vital for embryo and fetal development, as it supplies essential nutrients needed for proper growth, organ formation, and overall health throughout pregnancy.
Pollutants, heavy metals, pesticides, and radiation can negatively impact fetal development, especially in the first trimester. These exposures may lead to birth defects, developmental delays, or pregnancy complications.
Research suggests that sexual activity following IUI may enhance conception chances. The natural prostaglandins in semen can:
- Improve blood flow to the pelvic region
- Create beneficial uterine contractions
- Support sperm movement toward the egg
- Aid cervical softening
While not mandatory, intimate relations after IUI can complement the procedure. Doctors often encourage couples to maintain normal intimate relations, as it helps:
- Reduce stress levels
- Maintain emotional connection
- Provide additional sperm for fertilisation
- Support overall treatment success
When following medical guidelines, intimate relations after IUI are generally safe. However, patients should wait at least 18–24 hours post-procedure before resuming sexual activity. This allows the cervix to close correctly and minimises any risk of infection. Some mild spotting during this period is normal, but patients should contact their fertility expert if they experience significant discomfort or unusual symptoms.
Since infertility is no longer seen as a social taboo and views about it are rapidly changing, IVF has become more and more accepted as a means of achieving parenting in India, a country that once placed a high priority on biological parenthood.
Age significantly impacts IVF success rates. Younger individuals generally have higher success due to better egg quality and quantity, while success rates decline after the mid-30s, especially over 40.
Talking about infertility has become more socially acceptable as a result of celebrities discussing their experiences with IVF. Normalized IVF: As a result of public conversations, IVF has become more transparent and is now a more common choice for couples.
When considering IVF, individuals should prioritize the clinic’s reputation, success rates, and the technology used. These factors significantly impact the chances of a successful outcome. Considering your age, history of pregnancy, lifestyle, and medical history are also important.
The number of IVF cycles needed to achieve pregnancy varies depending on factors such as the couple’s age, underlying infertility issues, and embryo quality. At Ferty9 Warangal, we maintain a consistent IVF success rate of 71%, meaning most patients successfully conceive in the first cycle. However, in some cases, factors like age and the cause of infertility may lead to the need for additional cycles, although this is rare given our high success rates.
Factors like inclusions in the base price, medication costs, additional procedures, success rates, clinic reputation and expertise, location and convenience, financing and payment plans, and insurance coverage should all be taken into account when comparing IVF costs among clinics in Warangal.
The environment of an embryology lab is meticulously controlled to ensure optimal conditions for embryo development. This includes maintaining precise temperature, humidity, and gas levels within incubators. Sterility is preserved through the use of laminar flow hoods and air filtration systems, preventing contamination and safeguarding the health of gametes and embryos.
Embryology labs have seen significant technological advancements, including PICSI (Physiological Intracytoplasmic Sperm Injection), which aids in selecting the healthiest sperm for fertilization. Another innovation is DFI (DNA Fragmentation Index) testing, which evaluates sperm DNA integrity, enhancing the success rates of fertility treatments by selecting better-quality sperm.
Emerging technologies are expected to further transform embryology. AI and machine learning could enhance embryo selection processes by analyzing large datasets. Stem cell research may enable the creation of gametes, and improved genetic screening could lead to more successful IVF outcomes. These advancements aim to increase precision and boost success rates in fertility treatments.
Embryology lab technicians often form emotional connections with patients, making their work both fulfilling and emotionally taxing. While they experience the joy of contributing to successful pregnancies, they may also share in the disappointment when treatments are unsuccessful. Balancing empathy with professional detachment is a key challenge in this field.
Embryologists support patients throughout the IVF process by explaining procedures related to gametes and embryo development. They may discuss reasons for previous IVF failures and suggest ways to improve future outcomes. Their expertise and compassionate communication help guide patients through an emotionally intense journey.
Assisted hatching (AH) is generally considered safe and beneficial in assisted reproductive treatments. However, there are some risks involved, such as potential embryo damage, increased chance of twinning, and interference with the natural hatching process. A common concern is accidental harm to embryonic cells during the procedure, which involves thinning or breaching the embryo’s outer shell.
No, assisted hatching does not guarantee pregnancy. While it may increase the chances of implantation and pregnancy in certain patients—such as those with previous IVF failures or embryos with thick outer shells—it does not ensure conception for everyone.
Yes, assisted hatching can be performed with frozen or thawed embryos. It may improve implantation outcomes, particularly when embryos are thawed after cryopreservation, as freezing can sometimes cause hardening of the zona pellucida.
No, assisted hatching is not recommended for all IVF patients. It is generally reserved for specific groups, such as women over 37, those with poor embryo quality, thick zona pellucida, or a history of failed IVF attempts. A fertility specialist can help determine whether AH is appropriate based on individual medical history and embryo assessment.
Several pre-IVF tests and evaluations are crucial for determining the best course of treatment and optimising the chances of success. These may include:
- Ovarian reserve testing (e.g., anti-Müllerian hormone levels, antral follicle count)
- Semen analysis
- Uterine evaluation (e.g., hysterosalpingogram, hysteroscopy)
- Genetic testing
- Screening for medical conditions that may impact fertility
Your fertility specialist will recommend the appropriate tests based on your circumstances.
Yes, undergoing IVF counselling before starting the treatment can be highly beneficial. Counselling sessions can help couples:
- Understand the emotional & psychological challenges associated with IVF
- Develop coping strategies to manage stress & anxiety
- Prepare for potential outcomes & make informed decisions
- Receive emotional support throughout the process
Counselling can improve overall well-being and increase the chances of a successful IVF outcome.
Body weight can significantly impact IVF outcomes. Both underweight and overweight/obese individuals may experience reduced success rates. Obesity can lead to hormonal imbalances, ovulation issues, and higher complication risks during the IVF process. On the other hand, being underweight can also affect ovulation and egg quality.
Maintaining a healthy body weight through a balanced diet & regular exercise can improve the chances of a successful IVF outcome.
The chances of IVF success can be influenced by underlying health conditions, such as endometriosis, polycystic ovarian syndrome (PCOS), or autoimmune disorders. These conditions can affect fertility and may require additional treatment or adjustments to the IVF protocol.
At Ferty9, empowering our patients with knowledge is crucial for their safety and well-being. Our doctors take the time to explain the IVF process, potential risks, and alternative options in detail, ensuring that our patients can make informed decisions about their care. We also provide ongoing support and counselling services to help our patients navigate the emotional and psychological challenges they may face during this journey.
Our commitment to patient safety is intertwined into every aspect of our operations. We have implemented comprehensive patient safety protocols, including thorough screening and evaluation, informed consent processes, medication management, infection prevention measures, and continuous monitoring. Our state-of-the-art facilities and advanced laboratory standards ensure a controlled and sterile environment, minimising the possibility of contamination or adverse events.
At Ferty9, we are committed to continuous quality improvement. Our laboratory implements stringent quality control measures, including regular testing and validation of equipment, reagents, and processes, to ensure consistent and reliable results. With strict adherence to safety protocols and employment of the latest and most advanced IVF techniques, we have improved the chances of successful outcomes.
Our highly experienced team of fertility experts is dedicated to providing world-class care and ensuring the utmost safety for our patients. We adhere to strict embryo handling, storage, and transportation protocols. Our staff undergo regular infection prevention and control training, and we employ advanced air filtration systems and strict cleaning and disinfection procedures.
We take infection control measures extremely seriously, implementing internationally accepted protocols to prevent the spread of pathogens and maintaining a sterile environment throughout our facilities. We employ advanced air filtration systems and strict cleaning and disinfection procedures. Additionally, our state-of-the-art laboratory adheres to the highest industry standards, utilising cutting-edge technology and rigorous protocols to maintain a controlled and sterile environment.
Age is a significant component that can influence the success rates of IVF treatments. Women under the age of 35 typically experience higher success rates due to a better ovarian reserve and healthier eggs. As women age, both the quantity and quality of their eggs decline, which can reduce the chances of conception and increase the risk of miscarriage. Similarly, male fertility can also be affected by age; studies suggest that men over 40 may experience reduced sperm quality, which can impact embryo development. Thus, the combined age of both partners plays a crucial role in IVF outcomes.
The chances of having multiples, such as twins, with IVF are higher compared to natural conception. This is because more than one embryo is often transferred to increase the likelihood of pregnancy. On average, the chance of twins from IVF is estimated to be around 30%. However, multiples can also occur naturally when a single fertilised egg splits into two embryos, resulting in identical twins. Fertility clinics carefully manage embryo transfers to balance success rates and minimize the risks associated with multiple pregnancies.
Genetic screening, particularly Preimplantation Genetic Testing (PGT), plays a vital role in improving IVF outcomes, including twin pregnancies. PGT allows specialists to screen embryos for chromosomal abnormalities before implantation. This helps identify the healthiest embryos with the highest potential for a successful pregnancy. While PGT does not directly increase the chances of twins, it ensures that if multiple embryos are transferred, they are genetically viable, thus supporting a healthy multiple pregnancy when twins occur.
Yes, achieving a successful IVF cycle is possible after multiple failures. Each cycle is unique, and various factors can contribute to the outcome. By identifying and addressing the causes of failure, making adjustments to the treatment plan, and exploring alternative options, many couples have gone on to achieve a successful pregnancy after multiple unsuccessful IVF cycles.
Seeking a second opinion from a different fertility specialist or clinic can be beneficial if you have experienced multiple failed IVF cycles. A fresh perspective and a different approach may help identify potential issues or factors that were previously overlooked. Additionally, different clinics may have varying success rates or specialised expertise that could improve your chances of success. Learn more about when to consult a new fertility specialist for second opinion after failed ivf.
While IVF is generally considered safe, undergoing multiple cycles can increase certain risks. These may include:
Ovarian Hyperstimulation Syndrome (OHSS): Repeated ovarian stimulation can increase the risk of OHSS, a potentially serious condition characterised by ovarian enlargement and fluid accumulation.
Multiple Pregnancies: The risk of multiple pregnancies (twins, triplets, etc.) increases with each IVF cycle, which can lead to complications for both the mother and babies.
Emotional and Financial Strain: Multiple IVF cycles can take a significant emotional and financial toll, leading to stress and anxiety.
Mental health is essential for the success of IVF treatments. Chronic stress, anxiety, and depression can impact fertility and IVF outcomes. Stress can affect hormone levels, ovulation, and embryo implantation. Additionally, the emotional strain of undergoing IVF can take a toll on mental well-being, further impacting the chances of success.
Undergoing multiple IVF cycles can be an isolating experience, as it can be challenging for others to understand the emotional and physical toll fully. Here are some strategies to help cope with feelings of isolation:
• Joining an online community or a support group for people going through similar experiences can provide a sense of connection and understanding.
• Communicate openly with your partner, family, and close friends about your feelings and experiences.
• Seeking counselling or therapy can provide you with a safe space to express your emotions and receive professional guidance in coping with the challenges of repeated IVF cycles.
• Prioritising self-care activities that bring you joy and relaxation—such as hobbies, exercise, or spending time in nature—can help counteract feelings of isolation and promote overall well-being.
ఐయూఐ తర్వాత జరిగే సంభోగం వల్ల, గర్భం వచ్చే అవకాశాలు పెరుగుతాయని పరిశోధనలు చెబుతున్నాయి. వీర్యం లో ఉండే సహజమైన ప్రోస్టాగ్లాండిన్ల వల్ల:
- కటి ప్రాంతానికి రక్త ప్రసరణ మెరుగుపడుతుంది.
- ఉపయోగపడే గర్భాశయ సంకోచాలు కలుగుతాయి.
- వీర్య కణాలు అండం వైపు కదలడానికి సహాయపడుతుంది.
- గర్భాశయ ముఖద్వారం మెత్తబడుతుంది.
వైద్యులు తరచుగా జంటలను సాధారణ సన్నిహిత సంబంధాలను నిర్వహించమని ప్రోత్సహిస్తారు, ఎందుకంటే ఈ చర్య:
- ఒత్తిడిని తగ్గిస్తుంది.
- ఇద్దరి మధ్య అనుబంధాన్ని నిలుపుతుంది.
- ఫలదీకరణ కోసం అదనపు వీర్యాన్ని అందిస్తుంది.
- చికిత్స విజయవంతం కావడానికి సహాయపడుతుంది.
వైద్యుల సూచనలు పాటిస్తే, ఐయూఐ తర్వాత జరిగే సంభోగం సాధారణంగా సురక్షితమే. అయితే, ప్రక్రియ జరిగిన తర్వాత కనీసం 18-24 గంటలు వేచి ఉండాలి. ఇది గర్భాశయ ముఖద్వారం సరిగ్గా మూసుకోవడానికి మరియు ఇన్ఫెక్షన్లు వచ్చే ప్రమాదాన్ని తగ్గించడానికి సహాయపడుతుంది.
ఈ సమయంలో కొద్దిగా రక్తపు మరకలు కనిపించడం సాధారణం, కానీ ఎక్కువ నొప్పి లేదా అసాధారణ లక్షణాలు ఉంటే వెంటనే మీ వైద్య నిపుణుడిని సంప్రదించాలి.
Metabolic imaging is considered a non-invasive technique that does not involve direct manipulation or removal of embryonic cells. As such, it is generally regarded as a safe procedure with minimal risks to the embryo. However, as with any medical procedure, there may be potential risks associated with the specific imaging equipment or techniques used, which should be discussed with your doctor.
Yes, metabolic imaging is often used in conjunction with other assisted reproductive technologies and embryo assessment methods. It can complement traditional morphological evaluations, genetic testing, and other advanced techniques to provide a comprehensive assessment of embryo viability and developmental potential.
During a metabolic imaging procedure, embryos are typically cultured in a specialised medium containing specific markers or dyes that interact with their metabolic byproducts. Advanced imaging equipment, such as time-lapse incubators or confocal microscopes, is then used to capture and analyse the distribution and intensity of these markers within the embryos. The procedure itself is non-invasive and does not require any direct manipulation of the embryos. Patients may not notice any significant differences compared to standard IVF procedures.
When selecting the appropriate IVF treatment, several factors should be considered, including the patient’s age, ovarian reserve, the cause of infertility, and the desired outcome (e.g., single or multiple births). Additionally, the patient’s financial situation and the availability of fertility treatments in their local area may also influence the choice of IVF option. Patients must have open and honest discussions with their doctors to ensure they fully understand the benefits, risks, and potential outcomes of each IVF treatment option.
The duration of an IVF treatment cycle can vary and depends on the specific approach and the individual’s response to the fertility medications. Generally, a conventional IVF cycle can take approximately 4-6 weeks from the start of the menstrual cycle to the embryo transfer. However, the timeline may be shorter or longer depending on various factors, such as the patient’s response to stimulation, the number of eggs retrieved, and the time required for fertilisation and embryo development.
IVF treatments can sometimes be accompanied by various side effects, which may include:
- Ovarian Hyperstimulation Syndrome (OHSS): A condition where the ovaries become enlarged with fluid accumulation in the abdomen, which can cause discomfort and other symptoms.
- Mood Changes: Fertility medications can sometimes lead to mood swings, irritability, or depression.
- Bloating and Abdominal Discomfort: Some patients may experience bloating or discomfort in the abdominal area as the ovaries become stimulated and enlarged.
- Headaches: Some patients may experience headaches during the IVF treatment process.
- Breast Tenderness: The hormonal changes associated with IVF can cause breast tenderness or swelling.
Yes, Natural Cycle IVF can be a suitable option for women with irregular menstrual cycles. In this approach, the IVF treatment is carried out during the woman’s natural menstrual cycle without the use of fertility medicines to stimulate egg production. This can be beneficial for individuals who are unable to tolerate or respond well to fertility drugs or for those with very low egg reserve.
Success rates for fertility treatments vary significantly based on individual circumstances. For women under 35, IVF pregnancy success rates reach 60-70% across multiple cycles. IUI treatments typically show success rates of 10-16% per cycle. These rates decrease with age and depend on factors such as:
- Duration of infertility
- Type of fertility issue
- Overall health condition
- Number of treatment cycles attempted
Doctors conduct comprehensive fertility evaluations for both partners. For women, primary tests include:
- Blood hormone analysis
- Ultrasound imaging of reproductive organs
- Hysterosalpingogram to check fallopian tubes
- Ovarian reserve testing
Male fertility testing focuses on semen analysis and hormone evaluation to determine sperm health and production.
The fertility treatment journey typically begins with initial consultations and diagnostic testing. Patients undergo regular monitoring through blood investigations and ultrasounds. Treatment cycles may require:
- Daily medication administration
- Frequent clinic visits for monitoring
- Timing coordination for procedures
- Two-week waiting period after treatment
- Regular communication with the healthcare team
Most treatments span 2-3 weeks per cycle, with success often requiring multiple cycles.
To protect yourself from air pollution during IVF, use air purifiers, monitor air quality, eat antioxidant-rich foods, and wear masks when needed.
Yes, air pollution may lead to complications like miscarriage and preterm birth during pregnancy, so minimizing exposure is crucial.
Yes, prolonged exposure to air pollution can impact fertility over time due to chronic inflammation, oxidative stress, and hormonal imbalances. Understanding the relationship between pollution and fertility is essential for preserving reproductive health.
The IVF process typically takes 4 to 6 weeks. This includes the initial consultation, ovarian stimulation, egg retrieval, fertilisation, and embryo transfer. If frozen embryo transfer (FET) cycles are used, it might take an additional 2 to 4 weeks. The exact duration depends on the couple’s individual response and medical condition.
Before beginning IVF, a comprehensive evaluation of both partners is performed to identify fertility issues and design a personalised treatment plan. Common tests include hormone level assessments, blood tests, infection screenings, and uterine structure evaluations. These ensure optimal preparation and reduce complications during the IVF process.
In India, under the Assisted Reproductive Technology (ART) Regulation Act of 2021, IVF age limits are defined. Women are generally advised not to use their own eggs after age 45 due to reduced success rates and increased risks. For men, sperm usage is permitted between the ages of 21 and 55.
IVF success rates vary depending on factors such as a woman’s age, cause of infertility, embryo quality, number of embryos transferred, and lifestyle. Clinics and regions may report different rates, but generally, success rates with donor eggs range from 50% to 70% per cycle.
IVF is considered a safe procedure, but it may involve certain risks and side effects such as ovarian hyperstimulation syndrome, side effects from hormonal medications, risks during egg retrieval and anaesthesia, infection, multiple pregnancies, miscarriage, and emotional or financial stress. Discussing these factors with your fertility specialist is essential before proceeding with treatment.
Fertility awareness is critical for the next generation because it allows them to make educated reproductive decisions, which promotes better relationships and family planning in the future.
Hormonal imbalances, polycystic ovarian syndrome (PCOS), poor sperm quality, lifestyle variables such as obesity and stress, and delayed family planning are all common reproductive concerns among young people today.
Yes, infertility is on the rise among younger generations, owing mostly to delayed childbirth, lifestyle choices, increased obesity rates, and reproductive health disorders such as polycystic ovarian syndrome (PCOS) and endometriosis.
Fertility outreach has a substantial influence on global reproductive health by raising awareness of fertility concerns, offering information and resources, and increasing access to reproductive healthcare services. It creates a more supportive atmosphere for persons dealing with fertility issues, leading to better health results and stronger communities throughout the world.
Yes, almost 1 in 8 couples experience difficulties conceiving or maintaining their pregnancy. This is a worldwide reproductive problem that impacts both genders.
Fertility education has the potential to improve people’s conception and fertility journey by raising awareness, empowering individuals, improving pregnancy outcomes, assisting with planning, and helping them understand available treatment choices.
Couples who lack enough awareness or information regarding fertility may feel unprepared when attempting to conceive. Comprehensive fertility education and support can help bridge this gap and provide much-needed clarity.
Most couples who try for a year will eventually become pregnant. Although less often, it is possible to become pregnant in the first month or within six months. Patience and persistence, along with proper medical advice when needed, are key.
Yes, patients have the right to question the choices made by fertility clinics under India’s new ART legislation. The 2021 Assisted Reproductive Technology (Regulation) Act creates procedures for handling complaints and conflicts.
Patients should understand Regulation and Accreditation, Costs and Financial Planning, Rights and Protections, and the significance of informed consent before beginning assisted reproductive technology (ART) procedures under the new Indian regulations.
In a number of ways, the new ART legislation in India can increase the success rates of reproductive treatments. They also foster an atmosphere that encourages safety, quality, and ethical concerns, all of which can lead to increased fertility treatment success rates.
Patients should approach certified ART fertility clinics and banks that adhere to these requirements and remain updated on the latest rules to be ready for changes in ART regulations. Additionally, being aware of their rights and making financial plans might help them successfully negotiate the changing fertility healthcare.
AI can manage chronic conditions in women by providing personalised treatment plans, monitoring disease progression, and identifying potential complications early. AI algorithms can analyse vast data, including medical records, lifestyle factors, and genetic information, to predict the likelihood of developing or exacerbating chronic conditions. Doctors use this information to implement preventive measures, lifestyle modifications, and tailored treatment strategies, ultimately improving disease management and enhancing the quality of life for women living with chronic illnesses.
Women can advocate for using AI in their healthcare by staying informed about the latest developments and advancements in AI-driven healthcare solutions. They can engage with their doctors, ask questions about the integration of AI technologies, and express their interest in exploring AI-powered tools and resources. Additionally, women can support organisations and initiatives that promote the ethical and equitable development and implementation of AI in healthcare, ensuring that these technologies benefit all women, regardless of their background or circumstances.
AI can play a significant role in assisting with menopause management by providing personalised guidance and support. AI-powered virtual assistants and chatbots can offer tailored advice on managing menopausal symptoms, such as hot flashes, mood changes, and sleep disturbances. Additionally, AI algorithms can analyse data from wearable devices and other sources to monitor hormone levels, track symptom patterns, and recommend lifestyle modifications or treatment options to alleviate menopausal discomfort. By leveraging AI, women can better understand their unique menopausal experience and access personalised resources to navigate this transitional phase with greater ease and support.
AI contributes to improving maternal and foetal health during pregnancy in several ways. AI-powered monitoring systems can continuously track vital signs, foetal movements, and other indicators, enabling early detection of potential complications or abnormalities. AI algorithms can also analyse medical images, such as ultrasounds, to identify potential risks or developmental issues, allowing for timely interventions. Additionally, AI-driven predictive analytics can assess risk factors and provide personalised recommendations for prenatal care, nutrition, and lifestyle adjustments to promote a healthy pregnancy and optimal foetal development. By leveraging AI, doctors can provide more comprehensive and proactive care, ensuring the well-being of both the mother and the developing foetus.
When selecting a fertility clinic in India, consider factors such as the clinic’s reputation, success rates, accreditations, quality of facilities, and the expertise of the medical team. It’s also important to check whether the clinic has experience with international patients and offers comprehensive support services.
India offers several advantages for fertility treatments, including significantly lower costs compared to Western countries, access to advanced reproductive technologies, highly skilled medical professionals, and tailored services for international patients. This makes India a popular destination for fertility tourism.
IVF treatments in India are considerably more affordable than in countries like the United States, the United Kingdom, or Australia. This cost advantage makes India an attractive option for couples seeking high-quality fertility care at a fraction of the price found abroad.
Medical tourism agencies help international patients by connecting them with reputable fertility clinics in India. They assist with travel arrangements, accommodation, translation services, and coordination of medical appointments. These agencies streamline the process and offer support throughout the fertility journey, making the experience more comfortable and hassle-free.
Oil-based lubricants are generally not recommended when trying to conceive naturally. These lubricants can potentially damage sperm cells, disrupt the vaginal pH balance, and increase the risk of infections. Water-based or fertility-friendly lubricants are typically safer options for couples attempting to conceive.
Yes, lubricants can potentially impact the success of fertility treatments. Some lubricants may contain ingredients that could interfere with sperm function, embryo development, or implantation. It is best to consult with a fertility specialist about lubricant use during treatment.
Vaginal pH plays a crucial role in fertility outcomes. A healthy vaginal pH ranges between 3.8 and 4.5, which helps protect against infections and supports sperm survival. Many lubricants can alter this balance, making the environment less favorable for conception. It’s important to choose pH-balanced, fertility-friendly lubricants to avoid disrupting vaginal health.
While occasional use of lubricants is unlikely to cause long-term fertility problems, frequent or prolonged use of certain types may contribute to issues. Some lubricants can disrupt the vaginal microbiome, cause inflammation, or affect hormone balance. If you’re facing fertility challenges, it’s wise to consult your doctor about suitable alternatives.
Yes, countries like Sweden and Japan have implemented effective policies such as generous parental leave and lifting birth restrictions for families to boost birth rates and support family life. These strategies are examples of how different nations have addressed the challenges associated with low fertility rates by country.
A population decline can impact India’s global position by potentially reducing its labor force and economic growth, which might affect its competitive edge and influence on the global stage.
Immigration can help mitigate labor shortages and demographic challenges, but it requires effective integration policies to ensure that immigrants contribute positively to the economy and society.
A declining birth rate could lead to reduced demand for educational services, potentially resulting in fewer schools and resources. However, it presents an opportunity to focus on enhancing the quality of education.
The fertility rate and fertility ratio are related but distinct demographic measures. The fertility rate, or the total fertility rate (TFR), is the average number of children a woman would have during her reproductive years, assuming that age-specific fertility rates remain constant. It measures the level of fertility in a population. On the other hand, the fertility ratio, or the general fertility rate (GFR), is the actual number of live births per 1,000 women of childbearing age (typically between 15 and 49 years old) in a given year. It measures the current level of fertility in a population.
There is no universally accepted “best” fertility rate for a country, as it depends on various factors, including economic development, social policies, and cultural norms. However, a fertility rate of around 2.1 is generally considered the replacement level fertility, where a population replaces itself from one generation to the subsequent one without experiencing growth or decline. Fertility rates below 2.1 can lead to an aging population and potential labor shortages, while those above 2.1 can strain resources and potentially hinder economic development.
Urbanization has played a significant role in India decline in fertility rates. As more people migrate from rural areas to urban centres, they tend to adopt lifestyles and attitudes more conducive to smaller family sizes.
Pollution, especially air pollution and exposure to harmful chemicals, has been linked to potential impacts on fertility rates in India. Several studies have suggested that high levels of air pollutants, like particulate matter (PM2.5 to PM10), nitrogen oxides, and sulphur dioxide, can adversely affect reproductive health and fertility. These pollutants can contribute to oxidative stress, hormonal imbalances, and reduced sperm quality in men, as well as increased risk of miscarriages, preterm births, and birth defects in women.
Yes, irregular cycles can influence ovulation, making it difficult to determine when you are most fertile. Moreover, if you have irregular periods, it may be more difficult to become pregnant since you do not release an egg (ovulate) on a regular basis.
Foods that can help with ovulation include beans, lentils, seafood, leafy greens, eggs, fruits and vegetables, nuts and seeds, and whole grains.
It is not safe to take herbal supplements to increase ovulation without consulting a doctor. Some herbs may interact with other drugs or supplements, causing unexpected hormonal consequences.
If you’ve been trying to conceive for more than a year without success, it may be time to consult a doctor about ovulation concerns. Irregular cycles, missed periods, or symptoms of hormonal abnormalities are all reasons to contact a medical professional.
Yes, pregnancy is possible with hyperthyroidism when properly managed through medication and regular monitoring. Most women with controlled hyperthyroidism can conceive and carry a healthy pregnancy to term. Key steps include:
- Regular thyroid function tests
- Appropriate medication adjustments
- Close monitoring by doctors
Hypothyroidism can impact fertility by disrupting ovulation and menstrual cycles. However, with proper thyroid hormone replacement therapy, conception chances improve significantly. Most women achieve normal fertility once their thyroid levels are optimised.
The primary tests for evaluating thyroid function include:
- TSH (Thyroid Stimulating Hormone)
- Free T4 (thyroxine)
These blood tests provide essential insights into how well the thyroid is functioning and guide appropriate treatment plans.
Yes, thyroid treatment during pregnancy is both safe and crucial. Most medications used to manage thyroid disorders are well-studied and safe for use during pregnancy. Regular monitoring ensures proper dosage adjustments throughout the pregnancy to protect both mother and baby.
Maintaining optimal thyroid health to support fertility includes:
- Consistent use of prescribed medications
- Regular check-ups with a healthcare provider
- Eating a balanced, nutrient-rich diet
- Avoiding foods that can interfere with thyroid function
Yes, research shows a strong link between thyroid disorders and increased risk of miscarriage. Women with unmanaged thyroid conditions face a higher likelihood of pregnancy loss. However, when thyroid levels are well-controlled, the risk is significantly reduced.
అటువంటి దేశాలు ఉన్నాయి. స్వీడన్ మరియు జపాన్ వంటి దేశాలు జననాల రేట్లను పెంచడానికి మరియు కుటుంబ జీవితానికి మద్దతు ఇవ్వడానికి ఉదారంగా, తల్లిదండ్రులు కాబోయే వారికి సెలవులు మరియు కుటుంబాల కోసం పెట్టుకున్న జననాల పరిమితులను ఎత్తివేయడం వంటి ప్రభావితమైన విధానాలను అమలు చేశాయి. తక్కువ జననాల రేట్లతో కూడిన సవాళ్లను వివిధ దేశాలు ఎలా పరిష్కరించాయో తెలుసుకోవటానికి, ఈ వ్యూహాలే ఉదాహరణలు.
మన దేశంలో జనాభా తగ్గిపోతే, ప్రపంచంలో మన స్థానం మారే అవకాశం ఉంది. ఎలాగంటే, పనిచేసేవారి సంఖ్య తగ్గిపోతుంది, దేశం ఆర్థికంగా ఎదగడం కూడా నెమ్మదిస్తుంది. దీనివల్ల ప్రపంచంలో మనకున్న పోటీతత్వం, మన మాట చెల్లుబాటు అయ్యే పరిస్థితి కూడా మారొచ్చు.
ఇతర దేశాల నుంచి మన దేశానికి వచ్చే వాళ్లు ఉంటే, ఇక్కడ పనిచేసేవారి కొరత తీరుతుంది, అలాగే జనాభాలో వస్తున్న మార్పుల వల్ల వచ్చే ఇబ్బందులు కూడా తగ్గుతాయి. కానీ, వలస వచ్చిన వారు మన ఆర్థిక వ్యవస్థకు మరియు సమాజానికి మంచిగా ఉపయోగపడేలా చూడటానికి సరైన విధానాలు ఉండాలి.
పిల్లలు పుట్టే రేటు తగ్గిపోతే, చదువు చెప్పే అవసరం కూడా తగ్గుతుంది. దానివల్ల స్కూల్స్ మరియు వాటికి కావలసిన వస్తువులు కూడా తగ్గిపోయే అవకాశం ఉంది. కానీ, ఇది చదువు యొక్క నాణ్యతను మెరుగుపరచడానికి ఒక మంచి అవకాశం ఇస్తుంది.
ఫెర్టిలిటీ రేటు అనేది ఒక మహిళ తన పిల్లలు కనే వయస్సులో సగటున ఎంత మంది పిల్లలను కంటుందో తెలియజేస్తుంది, ఇది టోటల్ ఫెర్టిలిటీ రేటు (TFR)గా పిలుస్తారు. ఫెర్టిలిటీ రేషియో లేదా సాధారణ సంతాన రేటు (GFR) అనేది ఒక నిర్దిష్ట సంవత్సరంలో, 15 నుండి 49 సంవత్సరాల మధ్య వయస్సు గల ప్రతి 1,000 మంది మహిళలకు ఎంత మంది శిశువులు జన్మించారో తెలియజేస్తుంది.
సాధారణంగా 2.1 సంతానోత్పత్తి రేటును ‘భర్తీ స్థాయి సంతానోత్పత్తి’గా పరిగణిస్తారు. ఇది ఒక తరం తమ సంఖ్యను తగినంతగా భర్తీ చేసుకునే స్థాయి. ఇది వృద్ధుల జనాభా మరియు శ్రామిక శక్తి సమతుల్యతను కాపాడేందుకు అవసరం. ఈ స్థాయికి మించి లేదా తక్కువ రేట్లు సమాజంపై ప్రభావం చూపవచ్చు.
పట్టణీకరణ వలన భారతదేశంలో చిన్న కుటుంబాల పట్ల అభిరుచి పెరుగుతోంది. పల్లె ప్రాంతాల నుంచి నగరాలకు వచ్చేవారు జీవనశైలిని మార్చుకుంటున్నారు, ఇది పిల్లల సంఖ్య తగ్గడానికి కారణమవుతుంది.
గాలి కాలుష్యం మరియు రసాయనాల ప్రభావం పునరుత్పత్తి ఆరోగ్యాన్ని దెబ్బతీస్తుంది. PM2.5, NOx, SO2 వంటి కాలుష్య కారకాలు శరీరంలో ఒత్తిడిని పెంచి హార్మోన్ల సమతుల్యతను దెబ్బతీస్తాయి. ఇది పురుషులలో వీర్య నాణ్యతను తగ్గించడమే కాకుండా, మహిళలలో గర్భస్రావం మరియు పుట్టుక లోపాలను పెంచుతుంది.
While everyones body responds differently, some people may notice improvements in a few weeks, especially in terms of menstrual regularity or energy levels. However, full benefits may take a few months as the body adapts to lower caffeine levels. Its important to combine caffeine reduction with other healthy lifestyle choices for the best results.
Yes, excessive caffeine intake during pregnancy may increase the risk of developmental issues and low birth weight. It is generally recommended to limit caffeine consumption to less than 200 mg per day to support a healthy pregnancy and fetal development.
Experts advise that women trying to conceive should limit their caffeine intake to under 200 mg per day. Men may also benefit from reducing caffeine, as high intake can potentially impact sperm quality and motility. Adopting moderate consumption habits can support reproductive health for both partners.
While moderate caffeine consumption may not drastically affect IVF outcomes, most fertility specialists recommend limiting intake during treatments. Reducing caffeine can help optimise hormonal balance and overall treatment success, so keeping it minimal is usually advised.
Hernia complications, such as compression or obstruction of the spermatic cord, testicular atrophy, or damage to the vas deferens, can directly impact sperm production, transport, and overall male fertility. Additionally, hernias can indirectly affect fertility by causing hormonal imbalances, chronic pain, or psychological distress.
Sometimes, small or asymptomatic hernias may be managed through a “watchful waiting” approach or supportive devices like hernia belts or trusses. However, for larger or symptomatic hernias, surgical repair is often recommended to prevent further complications and protect the reproductive organs.
Diagnosing a hernia includes a thorough physical examination, where the doctor may look for a bulge or lump in the affected area. Sometimes, doctors may perform additional tests, such as imaging techniques like ultrasound or CT scans, to confirm the diagnosis.
Untreated hernias can lead to various long-term effects on male fertility, including testicular atrophy, obstruction of the vas deferens, hormonal imbalances, chronic pain, and psychological distress. In severe cases, untreated hernias can potentially cause testicular infarction (death of testicular tissue) or testicular torsion, both of which can significantly impact fertility.
AndroMax stands out by offering a comprehensive and personalised approach to male fertility. It combines advanced diagnostics, tailored treatment plans, nutritional support, and lifestyle coaching to address the unique needs of each individual. Additionally, AndroMax seamlessly integrates with other fertility technologies, providing a holistic solution for couples seeking to conceive.
AndroMax addresses a wide range of male fertility issues, including low sperm count, poor sperm motility, abnormal sperm morphology, hormonal imbalances, genetic factors, and lifestyle-related contributors to infertility. By identifying and targeting the root causes, AndroMax aims to improve overall reproductive health and increase the chances of successful conception.
AndroMax uses cutting-edge fertility technology in several ways. It employs advanced diagnostic techniques, such as semen analysis, hormonal testing, and genetic screening, to identify potential fertility issues. In addition, AndroMax seamlessly integrates with other fertility technologies like IVF or IUI when necessary, enhancing the chances of successful conception and pregnancy.
At Ferty9, we understand that each individual’s fertility journey is unique. After conducting a comprehensive evaluation, our team of experts develops a personalised treatment plan tailored to each man’s specific needs and circumstances. This customised approach ensures that the interventions, lifestyle modifications, and nutritional support are optimised for maximum effectiveness.
Yes, it is safe to take vitamin supplements while trying to conceive. However, it’s important to consult with your healthcare provider to determine the right types and doses for your specific needs. Personalized guidance ensures that you are supporting your reproductive health effectively and safely.
Magnesium supports hormone regulation and reproductive health. It’s involved in various biochemical reactions that are crucial for ovulation in women and sperm production in men. Adequate magnesium levels can help create a more favorable environment for conception.
While most vitamins support fertility, excessive intake of certain vitamins, such as vitamin A, can negatively impact reproductive health. High doses may lead to toxicity and complications. Always adhere to recommended dosages and consult with a healthcare provider for safe supplementation.
Yes, stress and other lifestyle factors can influence how effectively the body absorbs and utilizes vitamins. High stress levels can disrupt hormonal balance and reduce the efficacy of fertility-supporting nutrients. Incorporating stress management techniques alongside proper nutrition can enhance fertility outcomes.
While the risk may be lower compared to long-term use, even short-term use of certain painkillers can potentially impact fertility. It’s essential to discuss any medication use with your doctor, especially if you are trying to conceive or maintain your fertility.
The time it takes for fertility to restore to normal after stopping painkillers can vary and depends on the type of medication, duration of use, and individual factors. Generally, it may take several menstrual cycles or months for the body to recover and return to its normal fertility levels.
If you are experiencing irregular menstrual cycles, difficulty conceiving, or other fertility-related issues, it’s important to discuss your concerns with a doctor. They may recommend tests or evaluations to assess your fertility and determine if painkillers or other factors are contributing to the issues.
Individuals with pre-existing conditions, such as endometriosis, polycystic ovary syndrome (PCOS), or other gynaecological issues, may be more susceptible to the potential fertility impacts of painkillers. It’s crucial to consult a doctor to understand the risks and explore alternative pain management strategies.
Yes, but only if you have an iron deficiency. Taking iron supplements can help resolve ovulatory issues and also prevent anemia once you are pregnant. However, its important to consult your doctor before starting any supplementation.
Yes, iron is one of the key nutrients influencing both fertility and fetal health. Iron deficiency has been strongly associated with high maternal morbidity, fetal deaths, and complications during childbirth. Managing iron levels is crucial for a healthy pregnancy.
Iron deficiency can lead to irregular or delayed menstrual cycles. In more severe cases, it might cause the body to stop menstruating altogether as a protective mechanism to prevent excessive blood loss.
While iron deficiency is more commonly associated with female fertility, it can also impact male fertility. Low iron levels can affect sperm count and function, making it harder for sperm to successfully fertilize an egg.
కరీంనగర్లో IVF క్లినిక్ను ఎంచుకునేటప్పుడు, క్లినిక్ యొక్క సక్సెస్ రేట్లు (విజయాల శాతం), దానికి ఉన్న గుర్తింపు మరియు సర్టిఫికెట్లు, వైద్య బృందం యొక్క నైపుణ్యం, అందుబాటులో ఉన్న సేవలు, వారు వాడే ఆధునిక సాంకేతికత, ఆసుపత్రి సౌకర్యాలు మరియు మీకు అనుకూలమైన ప్రదేశంలో ఉందా లేదా అనే వంటి అంశాలను పరిగణలోకి తీసుకోవాలి. ఇతర రోగుల సమీక్షలను (reviews) చదవడం మరియు నిపుణులతో నేరుగా మాట్లాడటం కూడా సరైన నిర్ణయం తీసుకోవడంలో మీకు సహాయపడుతుంది.
అవును, కరీంనగర్లో IVF చికిత్స ఖర్చు ఒక సైకిల్ నుండి మరొక సైకిల్కు మారవచ్చు. ఈ ఖర్చును ప్రభావితం చేసే అంశాలలో రోగి వయసు, వారి ఆరోగ్య చరిత్ర, సంతానలేమి రకం మరియు తీవ్రత, అవసరమైన మందులు, మరియు ICSI లేదా పిండం నిల్వ (embryo freezing) వంటి ఏవైనా అదనపు ప్రక్రియలు ఉన్నాయి.
అవును, ఒకవేళ IVF సైకిల్ విజయవంతం కాకపోయినా కొన్ని ఖర్చులు ఉంటాయి. వీటిలో డాక్టర్ కన్సల్టేషన్ ఫీజులు, నిర్ధారణ పరీక్షలు, మందులు, ల్యాబ్ ప్రక్రియలు మరియు ఎంబ్రియాలజీ సేవలకు అయ్యే ఖర్చులు ఉంటాయి. ఒకవేళ సైకిల్ విఫలమైతే, ఏ ఖర్చులు తిరిగి చెల్లించబడతాయి (refundable) మరియు ఏవి చెల్లించబడవు అనే వివరాలతో కూడిన పూర్తి కాస్ట్ బ్రేకప్ను క్లినిక్ను అడిగి తెలుసుకోవడం చాలా ముఖ్యం.
Yes, IVF can help treat male infertility, particularly in cases of low sperm count or motility. Using techniques like ICSI, a single healthy sperm is injected directly into an egg, overcoming many male-factor infertility challenges.
The best male infertility treatment depends on the cause. ICSI with IVF is effective for low sperm count and motility difficulties, while surgical treatments such as varicocelectomy or vasectomy reversal can address blockages. Medications and improved sperm retrieval procedures, such as microTESE, are potentially feasible choices for increasing fertility.
ICSI (Intracytoplasmic Sperm Injection) treats male infertility by directly injecting a single sperm straight into an egg, hence overcoming difficulties such as low sperm count, poor motility, and sperm quality. This method is especially effective for severe male factor infertility, such as non-obstructive azoospermia.
While there is no single “cure” for male infertility, many treatment options can effectively address underlying causes and significantly improve fertility. Advanced techniques like ICSI, IVF, and microTESE offer high success rates for couples facing male infertility challenges.
The cost of IVF for male infertility can change significantly depending on the clinic and the treatments required. On average, an IVF cycle can cost between INR 1.5 lakhs and INR 2 lakhs, with additional costs for medications, sperm retrieval techniques like ICSI, and embryo freezing.
The underlying etiology of asthenozoospermia determines the prognosis for the illness. Treatments for reversible conditions like varicocele or infections might enhance sperm motility. However, long-term or chronic difficulties may result from congenital conditions such as undescended testicles or hormonal imbalances.
Yes, keeping a healthy lifestyle can help lower the chance of developing reduced sperm motility, or asthenozoospermia. Avoid harmful habits, regular exercise, consume a well-balanced diet, control your stress, and schedule routine checkups with the doctor.
The condition known as complete asthenozoospermia affects 1 in 5,000 males, meaning that all of their sperm are immotile.
It takes three months for sperm to develop and get mature. The likelihood of a higher sperm count and motility is substantially higher after three months of the treatment.
ICSI allows for the selection of genetically healthy sperm, reducing the risk of passing on specific genetic or chromosomal abnormalities to the offspring. This is particularly beneficial for couples with a family history of genetic conditions or those who have experienced recurrent miscarriages due to genetic factors.
Yes, ICSI can be combined with other fertility treatments or procedures, such as preimplantation genetic testing (PGT) or assisted hatching. Your fertility specialist is the right person to determine the most appropriate combination of techniques based on your specific fertility challenges and needs.
The ICSI procedure is relatively quick, typically taking less than an hour. It is performed as part of the IVF cycle, and the recovery process is similar to that of a conventional IVF cycle. Most women can resume normal activities within a day or two after the egg retrieval procedure.
While ICSI can be beneficial for couples of various ages, there may be additional considerations for older women undergoing IVF. As a woman’s age increases, egg quality can decline, which may impact the success rates of ICSI. Your fertility specialist will evaluate your age, ovarian reserve, and overall health to determine the most appropriate treatment approach.
It is advisable to seek medical help if a couple has been trying to conceive for one year without success. However, if the woman is over 35 years old or if there are known fertility issues, it is advisable to see a fertility expert earlier. Early consultation can help identify underlying issues and initiate appropriate treatment sooner.
The success rates of infertility treatments depend on the underlying cause, the age of the couple, and the specific treatment method used. In general, the success rates for treatments like IVF and IUI range from 20% to 35% per cycle. However, success can vary significantly based on individual health factors, and a fertility specialist can provide a more personalised prognosis.
While infertility treatments are generally safe, there are some potential risks to be aware of. These may include ovarian hyperstimulation syndrome (OHSS), multiple pregnancies (especially with IVF or IUI), and the risks associated with surgical procedures. Close monitoring by a healthcare provider helps manage and minimise these risks effectively.
While there are no guaranteed natural treatments for primary infertility, some lifestyle changes and complementary therapies may improve fertility in certain cases. These include maintaining a healthy weight, managing stress, and incorporating fertility-boosting supplements or practices like acupuncture. However, it is essential to consult with a doctor before attempting any natural remedies, as they may not be suitable or effective for all infertility cases.
To minimise your exposure to microplastics, consider taking the following steps:
- Reduce plastic use: Choose reusable alternatives such as glass or stainless steel containers, and avoid single-use plastics.
- Proper waste disposal: Dispose of and recycle plastic waste responsibly to limit environmental contamination.
- Filter drinking water: Use a high-quality water filter capable of removing microplastics from your tap water.
- Avoid synthetic clothing: Opt for natural fibres like cotton or wool, as synthetic fabrics shed microfibres during washing.
- Support research and advocacy: Stay informed and support organisations combating microplastic pollution.
The long-term effects of microplastic exposure on fertility, particularly male fertility, are still being researched. However, chronic exposure may lead to cumulative impacts such as hormonal disruptions, oxidative stress, and genetic damage. These effects can potentially increase the risk of reproductive health issues over time.
Yes, microplastics in drinking water can negatively affect reproductive health. When ingested, microplastics can accumulate in organs, including the reproductive system. Research has linked them to hormonal imbalances, decreased sperm quality, and other fertility-related health concerns in both men and women.
Hormones play a crucial role in fertility by regulating ovulation, preparing the uterine lining, supporting early pregnancy, and influencing sperm production. Balanced levels of key hormones such as estrogen, progesterone, testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) are essential for successful conception and maintaining a healthy pregnancy.
A hormone imbalance occurs when there is too much or too little of one or more hormones in the body. This imbalance can cause a range of health issues, including infertility, irregular menstrual cycles, weight fluctuations, mood swings, and fatigue. Common causes include stress, poor diet, certain medical conditions, and natural ageing. Addressing these imbalances is key to restoring overall health and fertility.
Lifestyle changes can significantly improve hormonal health. Eating a nutrient-rich, balanced diet, exercising regularly, managing stress through techniques like yoga or meditation, getting quality sleep, and avoiding smoking and excessive alcohol consumption can help regulate hormones. Natural remedies such as herbal supplements and acupuncture may also offer support. Must Read: Lifestyle Options to support female infertility treatment.
Preventing hormonal imbalances involves adopting a healthy and proactive approach to overall wellness. This includes regular physical activity, a balanced diet, effective stress management, and avoiding endocrine disruptors. Routine health check-ups and timely medical intervention for symptoms of imbalance can help maintain hormonal health and support reproductive function.

Egg abnormalities can arise from various factors, including:
- Advanced maternal age
- Hormonal imbalances
- Genetic disorders
- Ovarian conditions (e.g., PCOS, endometriosis)
- Environmental factors (e.g., smoking, exposure to toxins)
- Underlying medical conditions (e.g., autoimmune disorders, cancer treatments)
While medical treatments like fertility medications and assisted reproductive technologies can help address egg quality issues, adopting a healthy lifestyle can also improve egg quality. Factors like maintaining a healthy weight, reducing stress, quitting smoking, and following an antioxidant-rich, balanced diet can positively impact egg quality.
The antral follicle count (AFC) is a measure of the number of small follicles present in the ovaries during the early follicular phase of the menstrual cycle. A higher AFC is generally associated with more available eggs, better egg quality and better ovarian reserve, indicating overall egg quality.
Some studies propose that acupuncture may help improve egg quality and increase the possibility of successful conception. Acupuncture is believed to promote better blood flow to the ovaries, regulate hormone changes, and reduce stress levels, which can potentially contribute to improved egg quality.
Generally, women can return to normal activities within 1 to 2 days after a hysteroscopy. Some may experience light spotting or mild cramping, but these symptoms usually resolve quickly, allowing for a swift recovery.
Yes, anesthesia may be used during a hysteroscopy to reduce discomfort. Depending on the procedure and patient needs, either local anesthesia to numb the cervix or general anesthesia (sedation) may be administered.
A doctor may recommend a hysteroscopy to investigate symptoms such as abnormal uterine bleeding, pelvic pain, or challenges with conception. The procedure allows for direct examination of the uterus to identify and sometimes treat potential issues.
While hysteroscopy is generally safe, there are some risks, including infection, heavy bleeding, damage to the cervix, or perforation of the uterus. These complications are rare, and the procedure is typically well tolerated.
Mild discomfort or pain may be felt during a hysteroscopy, particularly during cervical dilation or biopsy. However, this discomfort is usually short-lived and manageable with medication if needed.
Yes, specific exercises should be avoided or modified during IVF treatment. High-impact exercises (running, jumping, or intense aerobic activities) may be discouraged during certain stages of the IVF process, as they can potentially increase the risk of ovarian torsion or cause discomfort. Additionally, exercises that involve excessive abdominal crunches or twisting movements may be contraindicated after embryo transfer, as they can potentially dislodge the embryo. Consulting with your fertility specialist and following their recommendations for safe and appropriate exercise routines during each stage of the IVF process is crucial.
Yes, over-exercising or engaging in excessive physical activity can affect IVF success rates. Excessive exercise can lead to hormonal imbalances, disrupted menstrual cycles, and decreased fertility. It can also increase stress levels, disrupt sleep patterns, and negatively affect overall well-being, all of which can potentially reduce the chances of successful IVF treatment. It is crucial to strike a balance and avoid over-exercising during the IVF process.
If you have been leading a sedentary lifestyle, avoiding starting an entirely new and intense exercise routine during IVF treatment may be best. Sudden changes in physical activity levels can disrupt hormonal balance and increase stress levels, which may negatively impact IVF outcomes. However, it is generally safe to gradually incorporate gentle forms of exercise, such as walking, light stretching, or gentle yoga, during the IVF process, especially if you have been previously inactive.
Yes, exercise can potentially improve the success rates of IVF for women with fertility issues, such as polycystic ovary syndrome (PCOS), endometriosis, or hormonal imbalances. Regular physical activity can help regulate hormones, manage weight, reduce inflammation, and improve overall health, positively impacting fertility and IVF outcomes. However, it is essential to note that the benefits of exercise may vary and depend on the specific fertility issue and individual circumstances. Consulting with a fertility specialist and following their recommendations for appropriate exercise routines is crucial.
First-trimester screening is typically performed between weeks 11 and 14 of pregnancy, as this window provides the most accurate results for assessing potential risks and concerns.
First-trimester screening is not designed to detect all potential conditions or abnormalities. It primarily evaluates the risk of certain chromosomal abnormalities (Down syndrome, trisomy 18 & trisomy 13). However, it may also identify other potential concerns related to the baby’s growth and development.
Discuss your options with your doctor if you missed the optimal first-trimester screening window. They may recommend alternative screening methods, such as second-trimester screening or diagnostic tests, to assess potential risks and concerns.
If you have concerns or are unsure about the results of your first-trimester screening, it’s essential to discuss them with your doctor. They can provide further explanation, counselling, and guidance on the next steps, including additional testing or specialised care.
Women can identify ovulation through several physical signs. The most reliable indicators include a change in cervical mucus consistency, mild cramping on one side of the abdomen, and a slight rise in basal body temperature. Using ovulation prediction kits provides additional confirmation of approaching ovulation.
Daily tracking provides the most accurate picture of fertility patterns. Women should monitor their signs throughout their entire cycle, with particular attention to the following:
• Temperature readings each morning
• Cervical mucus changes daily
• Physical symptoms as they occur
Research indicates that women typically release one egg per menstrual cycle. While multiple eggs can be released within a 24-hour period, true multiple ovulations across different days in the same cycle are extremely rare.
Pregnancy is possible on the day of ovulation, but the chances are higher during the two days before ovulation. The egg remains viable for about twenty-four hours after release, while sperm can remain vital for up to five days in the female reproductive tract. This creates the optimal fertile window for conception.
IUI and IVF are two important assisted reproductive technologies that help women get pregnant in cases of infertility issues. In both cases, the woman receives medicine that promotes the development of several egg-producing follicles in her ovaries. In IUI, after the ovulation induction, the sperm is placed inside the uterus for fertilization to take place. In IVF, the eggs, after achieving the desired development, are taken out of the uterus, and fertilization of the egg and sperm occurs in the laboratory.
It frequently takes more than one treatment cycle to conceive. Some people, meanwhile, will not become pregnant even after several attempts. Your doctor may suggest in vitro fertilization (IVF) if you have experienced many cycles of ineffective ovulation induction.
Monitoring follicular development and the ovulation process requires regular hormonal blood tests. Hormonal baselines, including Anti Mullerian Hormone (AMH), estrogen, and Follicle-Stimulating Hormone (FSH), are assessed before ovulation stimulation. The key hormones that are analyzed during this phase are:
- Estradiol (estrogen)
- Progesterone
- Luteinizing hormone
The TESA (Testicular Sperm Aspiration) procedure is a minimally invasive technique used to retrieve sperm directly from the testis. It is primarily employed in cases of azoospermia, where the individual is unable to produce sperm in the ejaculate.
Studies have shown that the success rates of TESA-IVF can be comparable to those achieved with conventional IVF using ejaculated sperm. However, the success rates can vary and depend on various factors, such as the underlying reason for infertility, the age of the individuals involved, and the medical team’s expertise.
The TESA procedure is generally considered safe and minimally invasive. Its potential side effects may include mild pain at the injection site, swelling or bruising in the scrotal area, infection (though rare), bleeding or haematoma formation, and temporary testicular tenderness or discomfort.
Most individuals experience a full recovery within 1–2 weeks, and they can resume normal daily activities and light exercise. However, doctors may recommend rest and limited physical activity for the first few days following the TESA to allow proper healing.
The success rates of fertility preservation methods can vary and depend on a myriad of factors, such as the woman’s age, the specific technique used, and the quality of the preserved eggs, embryos, or ovarian tissue. Generally, egg freezing (oocyte cryopreservation) has a success rate ranging from 30% to 60% for women under 35, while embryo freezing has a slightly higher success rate. However, consulting with a fertility specialist is essential to understand the specific success rates based on individual circumstances.
Age is an essential factor that can impact the success of fertility preservation methods. A woman’s fertility naturally declines with age, and the quality and quantity of eggs decrease over time. Generally, it is recommended to pursue fertility preservation at a younger age, ideally before the age of 35, to maximize the chances of successful conservation and future conception.
Premature ovarian failure (POF) is a condition where a woman’s ovaries become non-functional before the age of forty, leading to infertility. Fertility preservation can be beneficial for women with POF by allowing them to preserve their eggs or ovarian tissue before the onset of the condition. Doctors then use this preserved material in assisted reproductive technologies to conceive a child in the future.
Yes, secondary infertility can be caused by factors affecting either partner or an amalgamation of issues from both partners. Both individuals should undergo comprehensive fertility evaluations to identify any potential contributing factors.
Absolutely. Secondary infertility can be a symptom of various underlying health conditions, such as hormonal imbalances, autoimmune disorders, or chronic illnesses. Identifying and addressing these conditions is crucial for improving fertility outcomes.
Endometriosis (endometrial tissue grows outside the uterus) can cause scarring, adhesions, and inflammation, potentially leading to blocked fallopian tubes or impaired implantation. This can make it difficult to conceive and increase the risk of secondary infertility.
The success rates of secondary infertility treatments vary depending on the specific cause, the couple’s age, and the treatment method used. With advancements in assisted reproductive technologies, many couples have a good chance of conceiving with the appropriate treatment approach. It is crucial to discuss success rates with a fertility specialist based on individual circumstances.
Several lifestyle modifications can help protect and enhance male fertility. The most effective preventive measures include maintaining a healthy weight, avoiding exposure to excessive heat, limiting alcohol consumption, staying away from tobacco and recreational drugs, wearing loose-fitting undergarments, keeping mobile phones away from pockets, engaging in regular exercise without overexertion, and managing stress effectively.
Age significantly impacts male fertility, particularly after 40 years. Studies show that conception is 30% less likely for men over 40 compared to those under 30. This decline relates to decreased sperm quality, reduced testosterone levels, and potential DNA fragmentation in sperm cells.
Dietary habits directly influence sperm quality and production. Fresh fruits, vegetables, and whole grains support optimal fertility, while processed meats and high-fat dairy products may reduce sperm quality. Antioxidant-rich foods particularly benefit sperm health, while excessive soy intake might decrease sperm concentration.
Sperm production naturally varies over time. Many factors, including stress, illness, and temperature changes, can cause temporary fluctuations in sperm count. These variations are typically normal and don’t necessarily indicate fertility problems unless persistent changes occur.
Decreased facial or body hair may indicate low testosterone levels, which can be linked to male infertility. Additional symptoms may include atypical breast development, fatigue, soreness, swelling or a lump in the testicle area, and erectile dysfunction. If you notice any of these symptoms, it’s important to consult a healthcare provider for proper diagnosis and treatment.
Testosterone replacement therapy, which can be administered through pellets, injections, patches, or gels, may help alleviate the symptoms of low testosterone. However, altering testosterone levels without medical supervision can lead to serious health issues such as testicular atrophy, infertility, and reduced sperm count. Always seek guidance from a qualified physician before starting any treatment.
Managing weight, limiting alcohol consumption, avoiding smoking, reducing stress, and practicing safe sex can all positively impact male reproductive health. Eating a well-balanced diet rich in protein, iron, and adequate calories can also support healthy hair growth and fertility.
If you experience symptoms such as reduced hair growth, fatigue, unusual breast development, swelling or lumps in the testicles, or erectile dysfunction—or if you and your partner have been trying to conceive for over a year—it is essential to consult a medical professional. Early diagnosis and proper treatment can significantly improve outcomes.
IVF ప్రక్రియకు సాధారణంగా 4 నుండి 6 వారాల సమయం పడుతుంది. ఇందులో ప్రారంభ సంప్రదింపులు, అండాశయ ప్రేరణ, గుడ్డు సేకరణ, పిండ తయారీ మరియు బదిలీ దశలు ఉంటాయి. ఫ్రోజెన్ ఎంబ్రియో ట్రాన్స్ఫర్ (FET) చక్రం ఉండినట్లయితే, అదనంగా 2-4 వారాలు పడవచ్చు. మొత్తం వ్యవధి జంట యొక్క ఆరోగ్య పరిస్థితి మరియు చికిత్సకు వారి ప్రతిస్పందనపై ఆధారపడి ఉంటుంది.
IVF ప్రారంభించే ముందు, ఇద్దరు భాగస్వాములకు పూర్తి ఫెర్టిలిటీ మూల్యాంకనం అవసరం. ఇందులో హార్మోన్ల స్థాయిలు, రక్తపరీక్షలు, గర్భాశయ మూల్యాంకనం మరియు ఇన్ఫెక్షన్ల స్క్రీనింగ్ వంటి పరీక్షలు ఉంటాయి. ఈ పరీక్షలు గర్భాశయ ఆరోగ్యం మరియు గర్భధారణకు అడ్డంకులు ఉన్నాయా అన్న దానిపై స్పష్టత ఇస్తాయి.
భారతదేశం లో 2021 ART చట్టం ప్రకారం, IVF కోసం మహిళలకు గరిష్ట వయస్సు 45 సంవత్సరాలు (గుడ్లను స్వయంగా ఉపయోగించే వారు)గా సూచించబడింది. పురుషులు తమ వీర్యాన్ని 21 నుండి 55 సంవత్సరాల వయస్సులో ఉపయోగించవచ్చు. వయస్సు పెరిగేకొద్దీ IVF విజయ రేట్లు తగ్గుతాయి మరియు ప్రమాదాలు పెరుగుతాయి.
IVF విజయ రేట్లు వివిధ అంశాలపై ఆధారపడి ఉంటాయి: స్త్రీ వయస్సు, పిండ నాణ్యత, స్త్రీ మరియు పురుష ఫెర్టిలిటీ సమస్యలు, బదిలీ చేయబడిన పిండాల సంఖ్య, మరియు చికిత్స నిపుణుల అనుభవం. సాధారణంగా ప్రతి IVF చక్రానికి విజయ రేట్లు 40-60% ఉండవచ్చు, అయితే డోనర్ ఎగ్ IVF విజయ రేట్లు 50-70% వరకూ ఉండొచ్చు.
IVF సురక్షితమైన ప్రక్రియ అయినప్పటికీ కొన్ని దుష్ప్రభావాలు ఉండవచ్చు: అండాశయ హైపర్స్టిమ్యులేషన్ సిండ్రోమ్ (OHSS), ఇన్ఫెక్షన్లు, గర్భస్రావం, బహుళ గర్భధారణలు, మానసిక ఒత్తిడి మరియు హార్మోన్లకు సంబంధించిన దుష్ప్రభావాలు. చికిత్స ప్రారంభించే ముందు వీటిని మీ ఫెర్టిలిటీ నిపుణుడితో చర్చించడం ముఖ్యం.
మధుమేహం గర్భధారణను ప్రభావితం చేయవచ్చు. మధుమేహం ఉన్న గర్భిణీ స్త్రీలు అధిక రక్తపోటు, కంటి సమస్యలు, మూత్రపిండాల దెబ్బతినడం మరియు మాక్రోసోమియా వంటి ప్రమాదాలను ఎదుర్కొనవచ్చు. గర్భధారణ సమయంలో రక్తంలో చక్కెర స్థాయిలను నియంత్రించకపోతే పుట్టుకలో లోపాలు మరియు నెలలు నిండకమునుపే ప్రసవం జరగవచ్చు. వైద్యుల పర్యవేక్షణలో ఉండడం, చక్కెర స్థాయిలను నియంత్రించడం ద్వారా మంచి గర్భధారణ ఫలితాలు సాధ్యపడతాయి.
అవును, మీరు గర్భం దాల్చగలరు. అయితే, మధుమేహం ఉన్న పురుషులకు సంతానోత్పత్తి సామర్థ్యం మీద కొన్ని ప్రభావాలు ఉండొచ్చు. ఇది స్పెర్మ్ కౌంట్, ఆకృతి, కదలిక మరియు డిఎన్ఎ నాణ్యతను ప్రభావితం చేయవచ్చు. సరైన చికిత్స, ఆరోగ్యకరమైన జీవనశైలి, మరియు ఫెర్టిలిటీ నిపుణుల సహాయంతో గర్భధారణ అవకాశాలు మెరుగవుతాయి.
మధుమేహం ఉన్న భర్తతో గర్భం పొందాలంటే, ముందు రక్తంలో చక్కెర నియంత్రణపై దృష్టి పెట్టాలి. స్పెర్మ్ విశ్లేషణ చేయించుకొని, అంగస్తంభన సమస్యలు లేదా ఇతర కారణాలను గుర్తించాలి. అవసరమైతే IUI లేదా IVF వంటి చికిత్సల గురించి నిపుణులను సంప్రదించాలి. ఆరోగ్యకరమైన జీవనశైలి కొనసాగించటం కూడా కీలకం.
అవును, మధుమేహం పురుషుల స్పెర్మ్ నాణ్యతపై ప్రతికూల ప్రభావం చూపుతుంది. ఇది స్పెర్మ్ కౌంట్ తగ్గడం, ఆకృతి మారడం, డిఎన్ఎ డామేజ్ మరియు కదలిక తగ్గడానికి దారితీస్తుంది. అయితే, సమయానికి వైద్య సహాయం తీసుకుంటే మరియు అవసరమైతే సహాయక సంతానోత్పత్తి పద్ధతులను అనుసరించినట్లయితే, మంచి ఫలితాలు పొందవచ్చు.
Male partners often experience a combination of emotional and psychological stress during IVF. Common challenges include anxiety about the outcome, performance pressure, and feelings of helplessness. Balancing personal emotions while offering support to their partner can be particularly difficult.
Yes, several lifestyle changes can enhance IVF success and sperm quality. These include maintaining a nutritious diet, exercising regularly, managing stress, avoiding smoking and excessive alcohol, and getting adequate sleep. These changes can contribute positively to overall reproductive health.
Absolutely. It’s normal for male partners to feel overwhelmed by the emotional and physical demands of the IVF process. Seeking support through counseling or speaking with others who have gone through similar experiences can provide relief and helpful perspective.
Providing steady emotional and practical support is essential. Listen with empathy, be patient, and reassure her that you’re in this journey together. Helping with daily tasks and considering professional support when necessary can also make a meaningful difference.
Yes, reduced sperm count can be linked to gynecomastia and hormonal abnormalities. Hormonal imbalances, particularly an increased level of estrogen or a decreased level of testosterone, can affect sperm production and overall male fertility. It is advisable to consult a healthcare provider for proper diagnosis and treatment.
Gynecomastia is often a benign (noncancerous) condition associated with various hormonal changes. In many situations, the cause is unknown, and it is typically not indicative of a serious medical issue. However, it can sometimes be linked to underlying health conditions, so medical evaluation is recommended if it develops suddenly or is accompanied by other symptoms.
No, gynecomastia surgery does not assist with male fertility concerns. The procedure is primarily cosmetic and focuses on reducing excess breast tissue. If you’re considering surgery, it’s also important to factor in the gynecomastia treatment cost, as this can change depending on the complexity of the procedure and the clinic you choose.
ఈ సమస్య యొక్క ఫలితాలు దాని వెనుక ఉన్న అసలు కారణంపై ఆధారపడి ఉంటాయి. వరికోసెల్ లేదా ఇన్ఫెక్షన్ల వంటి నయం చేయగల సమస్యలకు చికిత్స తీసుకుంటే వీర్య కణాల కదలిక మెరుగుపడవచ్చు. అయితే, వృషణాలు వాటి సంచిలోకి దిగకపోవడం లేదా హార్మోన్ల అసమతుల్యత వంటి పుట్టుకతో వచ్చే సమస్యల వల్ల దీర్ఘకాలిక ఇబ్బందులు ఉండవచ్చు.
అవును, ఆరోగ్యకరమైన జీవనశైలిని పాటించడం ద్వారా వీర్య కణాల కదలిక తగ్గకుండా చూసుకోవచ్చు, తద్వారా ఆస్థెనోజూస్పెర్మియా వచ్చే అవకాశాన్ని తగ్గించుకోవచ్చు. చెడు అలవాట్లకు దూరంగా ఉండండి, క్రమం తప్పకుండా వ్యాయామం చేయండి, సమతుల్య ఆహారం తీసుకోండి, ఒత్తిడిని నియంత్రించుకోండి, మరియు డాక్టర్తో నియమితంగా ఆరోగ్య పరీక్షలు చేయించుకోండి.
పూర్తిస్థాయి ఆస్థెనోజూస్పెర్మియా (అంటే వీర్య కణాలన్నీ అస్సలు కదలకపోవడం) అనే సమస్య ప్రతి 5,000 మంది పురుషులలో ఒకరిని ప్రభావితం చేస్తుంది.
వీర్య కణాలు అభివృద్ధి చెంది, పరిపక్వం చెందడానికి మూడు నెలల సమయం పడుతుంది. కాబట్టి, చికిత్స ప్రారంభించిన మూడు నెలల తర్వాత వీర్య కణాల సంఖ్య మరియు కదలిక గణనీయంగా మెరుగుపడే అవకాశం ఉంది.
అవును, తగ్గిన వీర్యకణాల సంఖ్యకు గైనెకోమాస్టియా మరియు హార్మోన్ల అసాధారణతలతో సంబంధం ఉండవచ్చు. హార్మోన్ల అసమతుల్యత, ముఖ్యంగా ఈస్ట్రోజెన్ స్థాయి పెరగడం లేదా టెస్టోస్టెరాన్ స్థాయి తగ్గడం వంటివి వీర్యకణాల ఉత్పత్తిని మరియు మొత్తం మగవారి సంతాన సామర్థ్యాన్ని ప్రభావితం చేస్తాయి. సరైన నిర్ధారణ మరియు చికిత్స కోసం వైద్య నిపుణుడిని సంప్రదించడం మంచిది.
గైనెకోమాస్టియా చాలా సందర్భాలలో హానికరంకాని (క్యాన్సర్ కాని) పరిస్థితి, ఇది వివిధ హార్మోన్ల మార్పులతో సంబంధం కలిగి ఉంటుంది. అనేక పరిస్థితులలో, దీనికి కారణం తెలియదు, మరియు ఇది సాధారణంగా తీవ్రమైన వైద్య సమస్యకు సూచన కాదు. అయితే, ఇది కొన్నిసార్లు అంతర్లీన ఆరోగ్య సమస్యలతో సంబంధం కలిగి ఉండవచ్చు, కాబట్టి ఇది అకస్మాత్తుగా వృద్ధి చెందితే లేదా ఇతర లక్షణాలతో కూడి ఉంటే వైద్య పరీక్ష చేయించుకోవడం మంచిది.
లేదు, గైనెకోమాస్టియా సర్జరీ మగవారి సంతానోత్పత్తి సమస్యలకు సహాయపడదు. ఈ ఆపరేషన్ ప్రధానంగా శారీరక రూపాన్ని మెరుగుపరచడానికి (కాస్మెటిక్) ఉద్దేశించబడింది మరియు అదనపు రొమ్ము కణజాలాన్ని తగ్గించడంపై దృష్టి పెడుతుంది. ఒకవేళ మీరు సర్జరీ గురించి ఆలోచిస్తున్నట్లయితే, గైనెకోమాస్టియా చికిత్స ఖర్చును కూడా పరిగణనలోకి తీసుకోవడం ముఖ్యం, ఎందుకంటే ఆపరేషన్ యొక్క క్లిష్టత మరియు మీరు ఎంచుకున్న క్లినిక్ను బట్టి ఇది మారవచ్చు.
Though it’s not necessary that individuals always inherit hypogonadism, some genetic factors and autosomal conditions causing HH do play a role in the same.
Yes, stress can reduce your testosterone levels. While stress affects different organs and hormone secretion, it also affects testosterone production. This is because stress generally activates the cortisol hormone, which suppresses the activity of other endocrine glands and reduces the body’s metabolism.
Sleep and testosterone production are closely intertwined. A study suggests that regularly not getting enough sleep can cause a young man’s testosterone to drop as much as it would if he were 10 to 15 years old. Lack of enough sleep can reduce testosterone levels by 10-15%. It can also increase cortisol levels and reduce testosterone secretion in your body.
Hypogonadism refers to a condition where males do not secrete enough testosterone and, therefore, can be considered to be infertile or less fertile, depending on their testosterone levels.
Testosterone levels must be checked once in 6-12 months or as prescribed by your healthcare professional while on therapy.
Though not always, hypogonadism is sometimes associated with other diseases like Kallmann’s syndrome – an abnormal condition of the pituitary. Moreover, tumors or certain injuries can also cause low testosterone.
Ovulation predictor kits (OPKs) are generally accurate in detecting the luteinising hormone (LH) surge that precedes ovulation. However, the correctness of the results can vary based on the timing of testing, medication use, and individual variations in hormone levels. Using OPKs in conjunction with other tracking methods is recommended for increased accuracy.
Yes, stress can impact your fertile window and overall fertility. High stress levels can disrupt the delicate hormonal balance required for ovulation and can lead to irregular or missed cycles. Managing stress through techniques like exercise, meditation, or counselling can help optimise fertility.
Tracking your fertile window can be more challenging if you have irregular menstrual cycles. In such cases, its recommended to use a combination of tracking methods, such as ovulation predictor kits, basal body temperature monitoring, and cervical mucus observations. Additionally, consulting a gynaecologist can guide you in identifying your fertile days and addressing any underlying issues.
While the chances of conception are highest during the fertile window, it is still possible to conceive outside this timeframe, although the likelihood is significantly lower. Sperm can live in the female’s body for up to five days. On the other hand, the egg can remain viable for up to 24 hours after ovulation. However, the probability of successful fertilisation decreases the further away from the fertile window.
If you need help to identify your fertile days despite consistent tracking, it’s advisable to consult with a doctor. They can evaluate your menstrual cycle, perform necessary tests, and provide guidance on alternative tracking methods or fertility treatments if needed. Additionally, seeking support from a fertility specialist can be beneficial in addressing any underlying issues or concerns.
Men with a history of testicular maldescent should consult a healthcare provider for regular fertility evaluations. Early detection of potential fertility issues and timely intervention, including hormonal therapies or surgical options, can help preserve or improve fertility. Regular check-ups are key to ensuring reproductive health and addressing any complications that may arise over time.
In some cases, testicular maldescent may correct itself during the first few months after birth. However, if the condition persists beyond six months, it typically requires medical intervention. Surgical correction, usually through orchiopexy, is often recommended to reposition the testicle and reduce future fertility and cancer risks.
Signs that testicular maldescent may be affecting fertility include difficulty conceiving, noticeable changes in testicular size or position, and discomfort in the groin area. A physical exam, hormone tests, or imaging may be necessary to evaluate fertility impact and determine the best course of action.
Men with a history of testicular maldescent should undergo regular follow-up evaluations as advised by their healthcare provider. These check-ups are important for monitoring testicular function, detecting early signs of fertility issues, and ensuring overall reproductive health throughout life.
While yeast infections are more common in women, they can also occur in men. The exact prevalence of yeast infections in semen is not well-documented, but they are considered relatively uncommon compared to other genital infections.
A doctor can diagnose a yeast infection in semen through a physical examination and laboratory tests. These may involve collecting a sample of the yeast infection discharge or affected skin for microscopic analysis or culturing to identify the specific type of yeast present.
While some natural remedies, such as probiotics, may help restore an optimal balance of microorganisms in the body, it’s essential to consult a doctor before attempting to treat a yeast infection with natural remedies alone. In some cases, yeast infection medication prescribed by your doctor may be necessary.
Over-the-counter antifungal treatments, such as creams or suppositories, can effectively treat mild yeast infections in semen. However, it is essential to adhere to the instructions and seek a doctor’s advice if symptoms persist or worsen. In some cases, antifungal medications may be necessary.
If one partner has a yeast infection, doctors often recommend that both partners receive treatment to prevent re-infection. Your doctor can guide you and your partner on the appropriate treatment plan.
Reduced hair growth on the face or body can indicate low testosterone levels, which may be linked to male infertility. Other symptoms can include abnormal breast enlargement, fatigue, pain, swelling or lumps in the testicular area, and erectile dysfunction. If you notice any of these symptoms, it’s important to consult a doctor for accurate diagnosis and treatment.
Testosterone replacement therapy — delivered through pellets, injections, patches, or gels — can help reduce symptoms of low testosterone. However, altering testosterone levels without medical supervision can lead to serious health problems, including testicular shrinkage, infertility, and reduced sperm count. Always seek guidance from a qualified doctor before starting any treatment.
Maintaining a healthy weight, reducing alcohol consumption, quitting smoking, managing stress, and practicing safe sex can positively affect male reproductive health. Eating a balanced diet rich in protein, iron, and adequate calories can also support healthy hair growth and fertility.
If you experience symptoms such as reduced hair growth, fatigue, abnormal breast enlargement, swelling or lumps in the testicles, or erectile dysfunction — or if you and your partner have been trying to conceive for over a year without success — it’s essential to see a healthcare professional. Early diagnosis and appropriate treatment can significantly improve outcomes.
అవును, గవదబిళ్లలు వృషణాలను ప్రభావితం చేయగలవు. ఈ పరిస్థితిని ‘మంప్స్ ఆర్కైటిస్’ అంటారు. యుక్తవయస్సు తర్వాత గవదబిళ్లలు సోకిన పురుషులలో సుమారు 20-30% మందిలో ఈ సమస్య సంభవిస్తుంది. మంప్స్ ఆర్కైటిస్ అంటే గవదబిళ్లల వైరస్ రక్త ప్రవాహం ద్వారా ప్రయాణించి, వృషణాల కణజాలాన్ని ఇన్ఫెక్ట్ చేయడం వల్ల కలిగే ఒకటి లేదా రెండు వృషణాల వాపు.
అవును, వ్యాక్సినేషన్ (టీకా) ద్వారా గవదబిళ్లలను నివారించవచ్చు. గవదబిళ్లల వ్యాక్సిన్ సాధారణంగా తట్టు-గవదబిళ్లలు-రుబెల్లా (MMR) వ్యాక్సిన్లో భాగంగా ఇవ్వబడుతుంది. ఇది ఈ మూడు వైరల్ వ్యాధుల నుండి రక్షణ కల్పిస్తుంది.
అవును, గవదబిళ్లలకు వ్యాక్సిన్ ఉంది. గవదబిళ్లల వ్యాక్సిన్ సాధారణంగా తట్టు మరియు రుబెల్లా వ్యాక్సిన్లతో కలిపి MMR (తట్టు-గవదబిళ్లలు-రుబెల్లా) వ్యాక్సిన్గా ఇవ్వబడుతుంది. ఈ కాంబినేషన్ వ్యాక్సిన్ను పిల్లలకు క్రమం తప్పకుండా ఇస్తారు మరియు ఇది ఈ మూడు వైరల్ వ్యాధుల నుండి దీర్ఘకాలిక రక్షణను అందిస్తుంది.
MMR వ్యాక్సిన్ గవదబిళ్లలు, తట్టు, మరియు రుబెల్లాను సమర్థవంతంగా నివారిస్తుంది. సెంటర్స్ ఫర్ డిసీజ్ కంట్రోల్ అండ్ ప్రివెన్షన్ (CDC) ప్రకారం, MMR వ్యాక్సిన్ యొక్క రెండు డోసులు గవదబిళ్లలను నివారించడంలో సుమారు 88% ప్రభావవంతంగా ఉంటాయి. అయితే, దీని ప్రభావం వ్యక్తి వయసు, వారి వ్యాధి నిరోధక శక్తి, మరియు సమాజంలో ప్రచారంలో ఉన్న గవదబిళ్లల వైరస్ రకం (స్ట్రెయిన్) వంటి అంశాలపై ఆధారపడి మారవచ్చు.
Yes, you can, but it’s unlikely to yield accurate results. The hCG levels might still be too low for detection. Waiting at least 4–7 days after implantation bleeding increases the chances of an accurate result.
The answer depends on timing. While hCG begins to rise shortly after implantation, it takes several days to reach detectable levels. If you take a test too early, you might get a false negative. Testing after a few days of implantation bleeding is crucial for reliable results.
hCG levels start to rise within 2–4 days after implantation. However, it may take 7–10 days after implantation for hCG to reach levels detectable by most home pregnancy tests.
If you get a negative result 2 weeks after implantation bleeding, it’s worth considering the following:
Incorrect Timing: Miscalculation of implantation date.
Low hCG Levels: Rare conditions or early miscarriage.
If doubts persist, consult your doctor for further evaluation.
You can test for pregnancy as early as a few days after implantation, but for the most accurate results, its best to wait. Implantation typically occurs 6 to 12 days after conception. Taking a test too early may result in a false negative because your body needs time for the pregnancy hormone hCG to build up to a detectable level.
After implantation, hCG levels start to rise, doubling approximately every 48 to 72 hours. With a sensitive test, you might get a faint positive 1 to 2 days after implantation. However, a clear positive result on most standard home pregnancy tests is more likely 3 to 4 days after implantation, and a definite positive is almost certain around the time of your missed period (7-10 days after implantation).
If you experience implantation bleeding, which is light spotting that occurs when the fertilized egg attaches to the uterine lining, you should wait a few days before testing. Since implantation bleeding happens around the same time your body starts producing hCG, its best to wait at least 3-4 days after the bleeding stops to allow hCG levels to rise sufficiently for a reliable result.
First Response Early Result tests are known for their high sensitivity and claim to detect hCG up to 6 days before your missed period. Since implantation happens between 6 and 12 days after ovulation, you can potentially test with a First Response kit as early as a day or two after implantation, but results will be more reliable as you get closer to your missed period.
Pregnancy can be detected as early as 1-2 days after implantation with a highly sensitive blood test performed by a doctor. For a home urine test, it’s recommended to wait at least 3-4 days after implantation for a reliable result, and for the highest accuracy, it’s best to wait until the first day of your missed period (approximately 7-10 days after implantation).
Pink spotting can be a sign of early pregnancy, particularly if it occurs around the time your period is due. It is important to note that while pink spotting may occur in early pregnancy, it can also be caused by other factors, so it’s advisable to consult with a healthcare provider for confirmation.
Implantation bleeding is typically light pink or brown and occurs a few days before your expected period. It is usually lighter than a regular period and lasts for a shorter duration, typically 1-2 days. If you experience this, it is often an early sign of pregnancy.
Light spotting is common in early pregnancy and can occur due to hormonal changes or implantation. However, heavy bleeding similar to a period may require medical attention, as it could indicate complications such as a miscarriage or other pregnancy-related issues.
Brown period blood for a week usually indicates old blood that is being expelled from the body. It can be normal, especially at the start or end of your period. However, prolonged brown bleeding should be checked by a doctor to rule out any underlying issues.
While reputable clinics strive for transparency, some costs may not be immediately apparent. These can include:
- Anaesthesia fees for egg retrieval
- Additional monitoring or blood tests
- Costs for freezing and storing extra embryos
- Medications, which can vary based on individual needs
Always ask for a detailed cost breakdown and clarify what’s included in the quoted price.
Clinics with higher success rates, experienced medical staff, and advanced equipment may charge more. However, better quality care can lead to higher success rates and potentially fewer treatment cycles, which may reduce overall costs in the long run.
Yes, IVF costs can vary widely across clinics in Hyderabad. Factors influencing the cost include:
- Clinic reputation and success rates
- Location (clinics in prime areas may charge more)
- Technology and equipment used
- Experience of the medical team
- Additional services offered
It’s a good idea to compare quotes from multiple clinics and review what’s included in each package.
Additional services can increase the overall IVF cost. Typical prices in Hyderabad include:
- Embryo freezing: ₹25,000 to ₹50,000 (including the first year of storage)
- Annual storage fee: ₹20,000 to ₹25,000
- Frozen Embryo Transfer (FET): ₹50,000 to ₹60,000
- Preimplantation Genetic Testing (PGT): ₹27,000 to ₹35,000 per cycle
Early pregnancy symptoms typically appear around two weeks after embryo transfer, if implantation is successful.
Implantation bleeding is usually light pink or brown and much lighter than a regular period. It doesn’t get heavier over time, unlike menstrual bleeding, which typically starts light and becomes heavier with a dark red flow.
A blood test can detect very low levels of HCG and is more sensitive than a urine test. It can confirm pregnancy as early as 7 to 10 days after conception.
Yes, cramping can be an early sign of implantation. It usually occurs 7 to 10 days after fertilization and feels similar to mild menstrual cramps.
Yes, IVF can slightly increase the chance of an ectopic pregnancy, especially in women with pre-existing fallopian tube damage. However, with proper embryo transfer techniques and close monitoring, the risk can be significantly minimised. It is important to consult with your fertility specialist about any concerns prior to undergoing IVF treatment.
At around 6 weeks, signs of an ectopic pregnancy may include severe abdominal or pelvic pain, abnormal vaginal bleeding, and dizziness or fainting. These symptoms can indicate a medical emergency and require immediate attention. Early diagnosis and treatment are crucial to avoid complications.
Ectopic pregnancy is diagnosed through a combination of pelvic examinations, blood tests to measure human chorionic gonadotropin (hCG) levels, and imaging studies such as a transvaginal ultrasound. These methods help determine whether the pregnancy is located inside the uterus or elsewhere, such as the fallopian tubes.
Yes, in certain early-detected cases, ectopic pregnancy can be treated without surgery using medications like methotrexate. This drug stops the growth of the pregnancy and allows the body to absorb it naturally. However, if the pregnancy is more advanced or causing significant symptoms, surgical intervention may be necessary.
Depending on personal health assessments, lifestyle modifications, and necessary medical examinations prior to beginning treatment, IVF preparation can normally take a few weeks to several months.
You should anticipate a number of medical tests prior to IVF, such as blood investigations, semen analysis, pelvic ultrasound, infectious illness screening, hormonal evaluations, genetic screening, and ovarian reserve testing.
Common medical conditions that might affect IVF success include endometriosis, polycystic ovary syndrome (PCOS), and uterine abnormalities. Furthermore, male factor infertility (such as low sperm quantity or motility), poor egg quality, and advanced maternal age can all have a substantial impact on IVF success.
Examine your coping strategies, support network, and capacity to handle the anxiety and stress that come with the procedure to see if you’re emotionally prepared for IVF. You should also think about talking to a fertility counselor or mental health specialist about your concerns.
Yes, sometimes extra medicine costs, diagnostic test costs, embryo freezing costs, and costs for more complex procedures like ICSI are examples of hidden expenditures related to IVF treatments. Having a thorough cost breakdown is crucial in preventing unforeseen costs. However, you can be certain that the treatments at our Ferty9 fertility center don’t come with any hidden costs.
Yes, age-related factors can affect IVF costs in Tirupati since older patients may need more advanced treatments, additional medications, or more cycles, which would raise total expenditures.
The cost of IVF in Tirupati is greatly influenced by the quality of care since clinics that use more sophisticated technology and have greater success rates may charge more for their services. Personalized treatment plans and extensive patient assistance may also result in higher costs, but they may also improve overall results. Ferty9 Tirupati provides quality care with cutting-edge technology, experienced specialists, extensive services, affordability, and—above all—a supportive environment.
IVF complications might result in longer treatment cycles, more medical interventions, and monitoring, which can raise total expenditures.
Doctors generally recommend abstaining from alcohol consumption for at least three months before starting an IVF cycle. This period allows the body to recover and optimise fertility levels for a better chance of success.
Even occasional or moderate alcohol consumption can potentially impact IVF outcomes. It is best to avoid alcohol altogether during the IVF process to minimise any associated risks and maximise the chances of successful fertilisation and implantation.
Alcohol consumption can disrupt the delicate hormonal balance necessary for a successful IVF cycle. It may affect the timing of ovulation or medication responses, so it is essential to follow the prescribed schedule and avoid alcohol throughout the process.
While secondhand alcohol consumption is not as direct as drinking yourself, it’s still advisable to avoid environments with heavy alcohol exposure. Minimising stress and potential toxins can contribute to better outcomes during IVF treatment.
Alcohol consumption can negatively affect the success rates of frozen embryo transfers (FET) by altering hormone levels and reducing uterine receptivity. For the best chance at successful implantation and pregnancy, its important to abstain from alcohol before and after FET.
Patients should wait at least 9-14 days after embryo transfer before taking a home pregnancy test. However, the most reliable results come from blood tests conducted at the fertility clinic, which can detect even small amounts of pregnancy hormones.
Preparation for the first scan involves several key steps:
- Drink water as advised by the clinic
- Wear comfortable, loose-fitting clothing
- Bring all relevant medical documents
- Schedule the appointment for the morning hours
- Have a light breakfast
- Arrange for a support person if desired
Anxiety before the first scan is completely normal and experienced by many IVF patients. Doctors understand these concerns and are prepared to offer reassurance and support throughout the process.
Early pregnancy signs may include breast tenderness, mild cramping, fatigue, and changes in appetite. However, some patients experience no symptoms at all, which doesn’t indicate any problems with the pregnancy.
Preparing yourself mentally is as important as physical preparation before the test. Patients should discuss any concerns with the fertility team, get adequate rest the night before, and focus on staying calm and positive. Writing down questions beforehand can help make the most of the appointment.
For centuries, ayurvedic practitioners have utilized herbs such as Ashwagandha, Shatavari, and Guduchi to regulate hormones. Stress and anxiety can be reduced with the aid of Ayurvedic herbs, which helps to address hormonal abnormalities indirectly. High levels of stress can lead to increased cortisol production, disrupting hormone synthesis and control in women.
Yes, Ayurvedic herbs can be used with modern fertility treatments. These herbs can help address infertility issues in both men and women. Combining contemporary medications with Ayurvedic herbal remedies may offer patients more treatment options, reduce side effects, and enhance overall therapeutic outcomes.
Some herbal combinations may contain hazardous heavy metals like lead and mercury, which can lead to serious health issues, including infertility. Additionally, certain herbs may cause abdominal pain, discomfort, diarrhea, nausea, vomiting, headaches, belching, stomach upset, and loose stools.
To improve reproductive health and optimize the condition of sperm, egg, and the mothers womb, it is generally believed that a minimum of three months, with an ideal period of twelve months, is required to see significant results with Ayurvedic herbs.
If both tubes are blocked, the most effective way to get pregnant is through In Vitro Fertilization (IVF). This procedure bypasses the fallopian tubes completely by fertilizing your egg with sperm in the lab and then placing the healthy embryo directly into your uterus.
Yes, it is definitely possible for a woman with blocked fallopian tubes to get pregnant with medical help. Treatments like IVF are specifically designed to help women in this situation achieve a successful pregnancy.
Yes, you can still get pregnant naturally or through Intrauterine Insemination (IUI) if you have one open and healthy fallopian tube. An egg from either ovary can travel through the open tube to be fertilized. Your fertility specialist might monitor you to ensure everything is functioning well on the open side.
The most common way to diagnose blocked fallopian tubes is with a test called a Hysterosalpingogram (HSG). Its a special X-ray where a safe dye is used to see if your fallopian tubes are open or blocked. Tubes can also be tested by other methods like Sonohysterography (SSG) or diagnostic laparoscopy – discuss your options with your specialist.
IVF is a highly effective and successful treatment for blocked fallopian tubes. Since the blockage is the main issue preventing pregnancy, IVF’s method of bypassing the tubes directly addresses the problem, leading to very good success rates without the need for surgery.
లేదు. IVF చికిత్సలో ఉపయోగించే మందులు సహజమైన ఉష్ణోగ్రత సరళిని మారుస్తాయి కాబట్టి, BBT పర్యవేక్షణ ద్వారా అండం విడుదలను కచ్చితంగా అంచనా వేయలేము. అండాల పెరుగుదలను గమనించడానికి మరియు అండాలను బయటకు తీయడానికి (egg retrieval) సరైన సమయాన్ని నిర్ణయించడానికి డాక్టర్లు రక్త పరీక్షలు మరియు అల్ట్రాసౌండ్ పర్యవేక్షణపై ఆధారపడతారు.
పిండ బదిలీ తర్వాత ఉష్ణోగ్రతను ట్రాక్ చేయమని సిఫార్సు చేయబడదు, ఎందుకంటే ఇది అనవసరమైన ఒత్తిడికి కారణం కావచ్చు. ఈ దశలో ఉపయోగించే మందులు మీ ఉష్ణోగ్రత సరళిని ప్రభావితం చేస్తాయి, అందువల్ల ఈ రీడింగ్లు చికిత్స విజయాన్ని సూచించే నమ్మదగిన సూచికలు కావు.
అవును, IVF మందులు ఉష్ణోగ్రత సరళిని అనేక విధాలుగా గణనీయంగా ప్రభావితం చేస్తాయి:
• స్టిమ్యులేషన్ మందులు: శరీర ప్రాథమిక ఉష్ణోగ్రతను పెంచగలవు.
• ప్రొజెస్టెరాన్ సప్లిమెంట్స్: పెరిగిన ఉష్ణోగ్రత అలాగే కొనసాగేలా చేస్తాయి.
• ట్రిగ్గర్ షాట్స్: తాత్కాలికంగా ఉష్ణోగ్రతను పెంచుతాయి.
• సహాయక మందులు: సహజమైన ఉష్ణోగ్రత సరళిని కప్పిపుచ్చవచ్చు.
IVF సమయంలో BBT ట్రాకింగ్ తప్పనిసరి కాదు. కానీ కొంతమంది రోగులు చికిత్సకు తమ శరీరం ఎలా స్పందిస్తుందో అర్థం చేసుకోవడానికి ఇది సహాయకరంగా ఉంటుందని భావిస్తారు. చికిత్సా నిర్ణయాల కోసం డాక్టర్లు ప్రాథమికంగా రక్త పరీక్షలు మరియు అల్ట్రాసౌండ్స్ వంటి ఇతర పర్యవేక్షణ పద్ధతులపై దృష్టి పెడతారు.
కొంతమంది రోగులకు, ఉష్ణోగ్రతను ట్రాక్ చేయడం అనేది తమ చికిత్సపై ఒక నియంత్రణ మరియు భాగస్వామ్యం అనే భావనను అందిస్తుంది. అయితే, మరికొందరిలో ఇది అనవసరమైన ఆందోళనను పెంచవచ్చు. BBT పర్యవేక్షణ మీకు ప్రయోజనకరంగా ఉంటుందో లేదో నిర్ణయించడానికి మీ వ్యక్తిగత ఇష్టాయిష్టాలను ఆరోగ్య బృందంతో చర్చించాలని డాక్టర్లు సిఫార్సు చేస్తారు.
It’s essential to ask and understand the full egg-freezing price, storage fees, medication costs, and any package deals available.
For many, fertility preservation is worth the cost, especially when considering the flexibility it provides. Evaluate your long-term family goals and assess how freezing your eggs aligns with them.
After the initial procedure, ongoing costs are mainly for storage. The egg-freezing storage cost is usually billed annually, and you may also incur additional charges if more than one cycle is necessary.
The average cost of fertility preservation depends on the procedure. Generally, freezing eggs costs start around INR 150000 to INR 200000, and storage fees are extra. Remember to budget for medications and possible insurance options, which can influence the final cost.
ITP (Immune Thrombocytopenia) affects approximately 1–2 in every 1,000 pregnancies. It is an autoimmune condition where the immune system attacks platelets, which are crucial for blood clotting. During pregnancy, maternal antibodies can cross the placenta and potentially lower the baby’s platelet count, requiring close monitoring and medical care.
Yes, women with ITP can safely undergo IVF with appropriate medical supervision. When platelet levels are well-managed, IVF success rates are similar to those without ITP. Doctors closely monitor platelet counts, especially during egg retrieval and embryo transfer, to ensure safety throughout the process.
You can enhance IVF success with ITP by maintaining optimal platelet counts through medications, strictly following treatment protocols, undergoing regular blood count monitoring, and working closely with both fertility and haematology specialists. Additionally, adopting healthy lifestyle modifications recommended by your doctors can support better outcomes.
The main risks associated with ITP during pregnancy include:
- Increased bleeding tendency
- Potential need for additional medications
- Possible complications during delivery
- Risk of low platelet counts in newborns
- Need for specialised monitoring throughout pregnancy
Immediate medical attention is necessary if patients experience unusual bruising, bleeding gums, or excessive menstrual flow. Regular consultations should occur weekly during stimulation phases and more frequently approaching critical procedures or if platelet counts fluctuate significantly.
A healthy lifestyle can boost fertility but doesn’t guarantee it. While maintaining a nutritious diet, regular exercise, and effective stress management are beneficial, other factors like genetics, hormonal imbalances, and underlying medical conditions can also influence fertility outcomes. It’s important to approach fertility holistically and seek professional advice if needed.
It generally takes a few months for your body to return to its natural cycle after stopping hormonal birth control. Some women may conceive immediately, while others may take one or two cycles. If you’re unsure or have concerns about returning fertility, it’s a good idea to consult a healthcare provider.
Regular menstrual cycles are usually a positive indicator of ovulation, but they don’t guarantee fertility. Issues such as blocked fallopian tubes, low egg quality, or uterine abnormalities may still prevent conception even when cycles are consistent. Comprehensive evaluation by a fertility specialist may be necessary for an accurate assessment.
Yes, although it is uncommon, it is possible to get pregnant during your period. Sperm can live inside the female reproductive tract for up to five days. If you have a shorter cycle and ovulate soon after your period ends, fertilisation can occur. Using contraception consistently is the best way to prevent pregnancy if you are not trying to conceive.
While lifestyle changes alone may not cure menorrhagia, they can certainly help manage symptoms. Maintaining a healthy weight, exercising regularly, and managing stress can all contribute to more regular menstrual cycles. Some women find that dietary changes such as quitting smoking, reducing caffeine and alcohol intake, and limiting fast food consumption can also support better menstrual health.
While you can’t always prevent menorrhagia, you can take steps to reduce your risk. Regular medical check-ups, a healthy lifestyle, and addressing any underlying health issues promptly can help. Maintaining a healthy weight, managing stress, and following a balanced, iron-rich diet are all key strategies to support overall menstrual health and reduce the risk of heavy periods.
Untreated menorrhagia can lead to chronic anaemia, which often causes fatigue, weakness, and shor.
The success rates of IVF for couples with a history of tubal pregnancy can vary depending on the extent of tubal damage, the couple’s overall fertility status, and the specific IVF protocols used. However, studies have shown that with the advancements in IVF technology and the expertise of fertility specialists like those at Ferty9, couples with a history of tubal pregnancy can achieve pregnancy rates comparable to those without this condition.
Before starting IVF treatment, foreign couples should be aware of the following:
- Understand the IVF process and timeline: It’s essential to have a clear understanding of the various steps involved in IVF, such as ovarian stimulation, egg retrieval, embryo transfer, and the potential need for multiple cycles.
- Prepare for the financial commitment: IVF can be a significant financial investment, and foreign couples should research the costs, payment options, and any potential insurance coverage or financial assistance programs available.
- Seek support and counselling: Navigating the emotional and psychological aspects of infertility and IVF can be challenging, especially for foreign couples. Seeking support from a counsellor or support group can be invaluable.
- Communicate with the fertility clinic: Foreign couples should proactively communicate with the fertility clinic, including any language or cultural barriers, to ensure a smooth and successful treatment experience.
A tubal pregnancy is when a fertilised egg gets implanted outside the females uterus, typically in the fallopian tube, contrary to the normal pregnancy in which the fertilised egg implants in the uterus. Tubal pregnancies can be life-threatening if not diagnosed and treated promptly, as the growing embryo can rupture the fallopian tube, causing internal bleeding and other complications.
Tubal pregnancy is typically diagnosed through a combination of the following methods:
- Blood investigations: Detecting human chorionic gonadotropin (hCG) levels (a hormone produced during pregnancy) can help detect the presence of a pregnancy.
- Ultrasound: Transvaginal ultrasound is the primary diagnostic tool used to visualise the location of the pregnancy and determine if it is ectopic or within the uterus.
- Physical examination: A pelvic exam may reveal signs of a tubal pregnancy, such as tenderness or a mass in the fallopian tube.
- Medical history: Your doctor will collect information about the patient’s medical history, including any previous ectopic pregnancies or risk factors for tubal pregnancy.
Ovarian cancer is much less common in young women compared to older women. Most cases are diagnosed after menopause. However, it’s still important to be aware of the risk factors, especially if there is a family history or genetic predisposition.
Yes, taking combination birth control pills can reduce the risk of ovarian cancer. Your risk starts to decrease after using the pill for just 3 to 6 months and continues to lower the longer you use it. However, consult your healthcare provider to understand if it’s a suitable option for you.
Painful periods can result from a range of underlying causes, including hormonal imbalance, endometriosis, fibroids, adenomyosis, pelvic inflammatory disease, ovarian cysts, bleeding disorders, and structural abnormalities. A medical evaluation is necessary to determine the exact cause.
Period cramps, often known as dysmenorrhea, seldom result in issues that interfere with your ability to conceive. However, if an underlying medical condition is causing your painful periods, it could affect fertility and should be evaluated by a specialist.
While period cramps are usually not serious, they can sometimes indicate a more significant medical issue. If painful periods are caused by conditions like endometriosis or fibroids, prompt diagnosis and treatment are essential to prevent complications.
Mild menstrual discomfort is common, but if the pain is severe enough to interfere with daily activities such as work or school, it’s not considered normal. You should see a doctor for evaluation and proper management.
Vitrified eggs can be stored indefinitely in liquid nitrogen tanks at extremely low temperatures (-196°C or -320°F). While there is no definitive limit on the storage duration, current research suggests that eggs can be safely stored for at least 10-15 years without significant degradation in quality or viability.
Like any medical procedure, oocyte vitrification has potential risks and side effects. These may include ovarian hyperstimulation syndrome (OHSS) due to hormonal stimulation, risks associated with egg retrieval (such as bleeding or infection), and emotional or psychological impacts. However, these risks are generally considered low, and the procedure is safe when performed by experienced fertility specialists.
Yes, vitrified eggs can be thawed and used for in vitro fertilisation (IVF) or other assisted reproductive technologies when an individual is ready to pursue pregnancy. After thawing, fertility experts and technicians fertilise the eggs using conventional IVF techniques or intracytoplasmic sperm injection (ICSI), and the resulting embryos are transferred into the uterus, similar to a traditional IVF cycle.
It is generally recommended that men have their sperm health checked if they are experiencing fertility issues or planning to conceive. However, even in the absence of fertility concerns, men should have a baseline sperm analysis performed around the age of 30 or before attempting conception. Doctors recommend regular sperm health check-ups for men with known risk factors or medical conditions that could impact sperm production.
If you and your partner have been trying to conceive for more than a year without success (or six months if the woman is over 35), it is recommended to seek medical advice. Additionally, if you have known risk factors or medical conditions that could impact sperm production, it is advisable to consult a specialist for a detailed evaluation and appropriate treatment.
This primary male sex hormone is crucial in initiating and maintaining spermatogenesis. It is secreted by the Leydig cells in the testes in response to the hormone LH (Luteinizing Hormone). Testosterone not only supports sperm production but also helps in the development of male reproductive organs and secondary sexual characteristics.
Excessive alcohol intake can negatively affect sperm production and quality. Alcohol can disrupt hormonal balance, contribute to oxidative stress, and directly damage sperm cells. Moderate alcohol consumption may have minimal impact, but heavy or binge drinking can significantly impair sperm production and fertility.
Obesity can have a detrimental effect on male fertility and sperm production. Excess body weight can result in hormonal imbalances, such as increased oestrogen levels and decreased testosterone levels, impairing spermatogenesis. Additionally, obesity is linked with an increased risk of oxidative damage and inflammation, both of which can damage sperm cells and impair sperm quality.
Fertility counseling is helpful for people or couples who are having trouble getting pregnant, are receiving fertility treatments, or are considering adoption or surrogacy. It’s particularly useful for handling relationship difficulties, controlling emotional stress, and making well-informed judgments regarding complicated reproductive treatments.
Counseling sessions often take between forty-five minutes and an hour, depending on the needs of the clients and the counselor’s approach. In order to collect comprehensive background information, some initial sessions could take longer, but follow-up sessions are frequently shorter and more targeted.
Yes, both partners can attend counseling together, and it is often encouraged. Joint sessions help improve communication, address shared concerns, and strengthen emotional support between partners, fostering a more unified approach to navigating fertility challenges.
The number of counseling sessions needed can vary depending on an individual’s or couple’s needs. Some individuals or couples may require more sessions, especially if they are navigating complex emotions or undergoing multiple fertility treatments. The counselor will tailor the number of sessions based on progress and goals.
After three tries with IUI, fertility professionals usually advise a more thorough assessment of the underlying reasons for infertility.
Yes, age significantly affects IUI success rates. As women age, the quality and quantity of eggs decrease, which can impact the likelihood of successful fertilization and implantation.
Yes, IUI can be effective even in cases where there are no apparent fertility issues. This is especially true for couples who are experiencing infertility that cannot be explained or who have modest sperm motility or cervical mucus abnormalities.
For the best likelihood of success, 3 to 6 IUI cycles are usually advised; with each additional cycle, the success rate marginally increases, particularly for women under 35.
Depending on variables including age, sperm quality, and underlying reproductive problems, the likelihood of IUI success on the first attempt usually varies between 10% and 20%.
Although treatment can still be successful, IUI with just one mature follicle typically has a slightly lower success rate than multiple follicles.
While fertility apps offer an effective way to track ovulation, combining their use with methods like BBT or ovulation kits can improve accuracy.
Fertility apps are beneficial for most users but may not be sufficient for individuals with underlying fertility issues. Consulting a specialist is recommended for comprehensive care.
Many fertility apps are free and offer basic features such as tracking ovulation and menstrual cycles. However, premium versions or subscriptions often require payment and include advanced features such as detailed fertility predictions, personalized health insights, access to fertility experts, or integration with wearable devices for more precise tracking.
Fertility apps provide emotional support by offering insights, setting realistic expectations, and connecting users with communities for shared experiences.
Most fertility tests are non-invasive and carry minimal risks. However, procedures like HSG might cause slight discomfort or cramping. Always discuss potential risks with your doctor.
The time varies depending on the test. Blood tests typically take a few days, while more complex procedures like genetic testing may take weeks.
Preparation depends on the specific test. For example, some blood tests require fasting, while semen analysis may require abstinence for a few days. Your doctor will provide specific instructions.
In most cases, yes. Fertility tests aim to pinpoint the reasons behind infertility, whether it’s hormonal imbalance, structural issues, or low sperm quality.
The recommended frequency of STD testing depends on various factors such as your age, sexual activity, and risk level. Generally, it’s advisable to get tested at least once a year or whenever you have a new sexual partner. However, individuals with higher risk factors or those engaged in high-risk behaviours may require more frequent testing.
While there is no single foolproof method, a combination of strategies offers optimal STD prevention. These include consistent and correct condom use, regular STD testing, open communication with partners, vaccination when appropriate, practising mutual monogamy, avoiding the sharing of personal items, and exercising caution with alcohol or drug use.
Open conversations with sexual partners about STDs are crucial. They help share each other’s sexual health status, build transparency and trust, encourage discussions around preventive measures, and ensure that both individuals can make informed decisions and seek testing or treatment if needed.
Untreated STDs can result in serious and lasting health complications, including infertility, chronic pelvic pain, neurological damage, increased risk of certain cancers, organ damage, and potential transmission to newborns during pregnancy or childbirth. Early detection and treatment are essential for preventing these outcomes.
Genetic issues that can impact IVF success include abnormal karyotypes, balanced translocations, single-gene mutations, and sex chromosome abnormalities. Couples in consanguineous marriages may carry recessive genetic disorders. Additional factors such as mitochondrial DNA mutations, genetic syndromes affecting reproductive organs, and genetic causes of very low sperm count can also reduce the chances of successful conception and implantation.
Ferty9 employs advanced genetic screening techniques including Karyotyping, Extended Carrier Screening, and Whole Exome Sequencing. To address genetic challenges, Ferty9 offers Preimplantation Genetic Testing for Aneuploidy (PGT-A), Single Gene Disorders (PGT-M), and Structural Rearrangements (PGT-SR), allowing for the selection of genetically normal embryos to improve IVF outcomes.
Genetic screening in IVF is advancing rapidly. Future developments may include more comprehensive genetic panels, integration of artificial intelligence and machine learning for enhanced embryo analysis, and consideration of epigenetic markers to assess embryo viability. These innovations aim to further improve success rates and personalise fertility care.
At Ferty9, personalised treatment plans are designed based on detailed genetic screening results. Fertility experts collaborate with each couple to understand their unique challenges and customise a strategy. This may involve selecting the healthiest embryos through preimplantation genetic testing or applying targeted fertility treatments tailored to their specific genetic concerns.
Most specialist doctors prescribe at least 7–9 hours of quality sleep per night to support optimal fertility. Adequate sleep is essential for maintaining hormonal balance, regulating the menstrual cycle, and supporting ovulation. Poor or inconsistent sleep patterns can disrupt these processes and potentially reduce fertility.
Yes, improving sleep patterns can positively impact fertility. Quality sleep helps regulate essential reproductive hormones such as estrogen, progesterone, and luteinizing hormone (LH). Healthy sleep habits can enhance both male and female fertility outcomes by promoting overall reproductive health and reducing stress, a known factor in infertility.
Certain sleep disorders, such as sleep apnea, insomnia, and restless leg syndrome, can negatively affect fertility. These conditions can lead to hormonal imbalances, elevated stress levels, and disrupted ovulation or sperm production. If you suspect a sleep disorder, it’s important to consult with a healthcare provider for evaluation and treatment, especially when trying to conceive.
Yes, a single embryo transfer can result in a twin pregnancy, but the chances are very low. Compared to natural conception, there is a higher likelihood of identical twins forming from a single embryo during IVF. This occurs when the embryo splits into two, leading to identical twins from one transferred embryo.
Elective Single Embryo Transfer (eSET) involves transferring the highest quality embryo from a fresh or previous IVF cycle. By choosing to transfer only one embryo, the risk of multiple pregnancies is significantly reduced. Additional embryos can be frozen and used in later cycles, maintaining a comparable success rate without the risks associated with twin pregnancies.
eSET typically results in singleton pregnancies, which are associated with fewer medical risks compared to multiple pregnancies. When multiple fetuses are carried, the risk of complications such as gestational diabetes, preterm birth, placental issues, and preeclampsia increases. eSET helps reduce these risks, promoting safer maternal health and more manageable pregnancies.
The success rate of IVF with single embryo transfer (SET) is closely tied to a woman’s age. Women under 35 typically have a success rate of 40% to 50% per transfer. However, this rate drops significantly in older women, with those over 40 seeing success rates between 10% and 20% per transfer. As age increases, both the quantity and quality of eggs decline, affecting outcomes.
Lifestyle changes, ovulation induction, and scheduled sexual activity, along with treatments like IUI and IVF, are common options for managing unexplained infertility. These methods aim to improve fertility and overall health and may lead to successful pregnancies, especially in younger couples. Customising the treatment plan can increase success rates.
If you have been diagnosed with unexplained infertility, consult experienced fertility doctors for a thorough evaluation. Detailed fertility testing will help rule out underlying conditions. Less invasive treatments are usually recommended first, with IUI and IVF considered based on the couple’s needs and the doctor’s advice.
IVF success rates for unexplained infertility vary by age and individual health conditions. Generally, they are comparable to those of women with a healthy ovarian reserve, with success rates ranging from 40% to 50% per cycle. Cumulative success rates increase with multiple IVF cycles.
IUI typically has lower success rates than IVF but can still be an effective first-line treatment for unexplained infertility. When combined with ovulation-inducing medications, IUI success depends on factors like age, menstrual regularity, and the overall reproductive health of the couple.
Unexplained infertility refers to difficulty in conceiving despite normal fertility test results after a year of trying. Secondary infertility occurs when a couple who has previously conceived struggles to get pregnant again. It can result from age, medical conditions, or male factor infertility.
The ideal age to consider IVF can vary depending on the results of a basic fertility evaluation and any previous fertility treatments. Consulting a fertility specialist will provide personalized guidance and help determine the best time for you.
Egg quality plays a crucial role in IVF success. Higher-quality eggs are more likely to develop into healthy embryos, increasing the chances of successful implantation and pregnancy.
It is possible to improve egg quality in 30 days by consuming fertility-friendly foods, taking supplements, and modifying your lifestyle routines. Adopting a healthy diet, reducing exposure to toxins, and maintaining hormonal balance can enhance egg health and increase the chances of successful conception.
Yes, stress can affect egg quality by releasing chemicals like prolactin and cortisol, which can interfere with or prevent ovulation and decrease egg production. Chronic stress can disrupt hormonal function and reduce reproductive efficiency, making stress management an important part of fertility care.
Yes, supplements such as CoQ10, DHEA, folic acid, and antioxidants are commonly recommended to promote egg health and improve egg quality. These supplements help enhance cellular energy production and protect eggs from oxidative damage, contributing to better fertility outcomes.
Yes, egg freezing serves as an option for women with poor egg quality. Since age is closely related to egg quality, freezing eggs at a younger age allows women to preserve their reproductive potential. This process enables them to use their own healthy eggs later for conception when natural fertility may have declined.
The best age for optimal fertility is typically in your 20s or early 30s. During this period, women have the highest number and quality of eggs, making it easier to conceive. Fertility declines gradually with age, particularly after 35, when both egg quantity and quality start to decrease significantly.
Obesity can negatively impact egg quality by disrupting hormone levels, leading to insulin resistance and increased inflammation. These factors can interfere with normal ovulation and egg development, reducing the chances of successful fertilization and conception.
Yes, smoking can significantly impact egg fertilization. It affects hormone levels, reduces blood flow to the reproductive organs, and impairs egg development. These changes can reduce the chances of successful fertilization and increase the risk of infertility.
Alcohol consumption can disrupt hormone balance, affect ovulation, and impair reproductive functions. In women, it may interfere with the menstrual cycle and reduce fertility, while in men, it can lower sperm quality and count, making conception more difficult.
Sleep plays a crucial role in maintaining hormonal balance and overall health, both of which are essential for reproductive health. Poor sleep patterns can disrupt hormone production, negatively impacting ovulation, menstrual cycles, and fertility outcomes.
High caffeine intake has been linked to reduced fertility, increased risk of miscarriage, and lower birth weight. Excessive caffeine may negatively affect egg quality and implantation. Limiting caffeine consumption can help support reproductive health and improve fertility outcomes.
Yes, many women with endometriosis successfully conceive and carry pregnancies to term. The chances of pregnancy depend on various factors, including the severity of the condition, age, and overall reproductive health. Early diagnosis and appropriate treatment can significantly improve fertility outcomes.
Endometriosis affects fertility in approximately 30-50% of cases. The impact varies based on several factors, such as:
– Stage of endometriosis
– Age of the patient
– Duration of infertility
– Presence of other fertility factors
Success rates for IVF with endometriosis vary depending on individual circumstances, especially age:
Age Group
Under 35: 30-40%
35-40: 20-30%
Over 40: 10-20%
Laparoscopic surgery can improve natural conception rates by removing endometrial lesions and adhesions. Most women see improved fertility within the first year after surgery, particularly if the endometriosis is mild to moderate.
A combination of lifestyle modifications can help manage symptoms and support fertility treatments. These include:
– Regular exercise
– Stress management techniques
– A balanced anti-inflammatory diet
These changes can significantly affect symptom management and overall well-being.
అవును, సీడ్ సైక్లింగ్ శరీరంలో హార్మోన్ల సమతుల్యతకు మద్దతు ఇవ్వడం ద్వారా క్రమం తప్పిన నెలసరిని సరిచేయడంలో సహాయపడగలదు. గింజలలో ఉండే పోషకాలు ఈస్ట్రోజెన్ మరియు ప్రొజెస్టెరాన్ అనే హార్మోన్ల స్థాయిలను నియంత్రించడంలో దోహదపడతాయి. ఈ హార్మోన్లు నెలసరి చక్రంలో చాలా కీలక పాత్ర పోషిస్తాయి కాబట్టి, వాటి పనితీరు మెరుగుపడితే నెలసరి క్రమంగా వచ్చే అవకాశం ఉంటుంది.
సీడ్ సైక్లింగ్ వల్ల ఫలితాలు కనిపించడానికి పట్టే సమయం ఒక్కొక్కరి శరీరాన్ని బట్టి మారుతుంది. కొంతమంది మహిళలు కొన్ని నెలసరి చక్రాలలోనే (అంటే 2-3 నెలల్లోనే) నెలసరి క్రమంగా రావడం లేదా ఇతర లక్షణాలలో ఉపశమనం గమనించవచ్చు. మరికొందరికి మాత్రం కొన్ని నెలల పాటు (3-6 నెలలు లేదా అంతకంటే ఎక్కువ) క్రమం తప్పకుండా సీడ్ సైక్లింగ్ చేయాల్సి రావచ్చు. శరీరంలో హార్మోన్ల సమతుల్యత అనేది నెమ్మదిగా జరిగే ప్రక్రియ కాబట్టి, ఓపికగా మరియు క్రమం తప్పకుండా ఈ పద్ధతిని పాటించడం చాలా ముఖ్యం.
ఈ పద్ధతిని సరిగ్గా, సూచించిన మోతాదులో గింజలను తీసుకుంటూ పాటిస్తే సాధారణంగా ఎలాంటి ప్రమాదం ఉండదు మరియు సురక్షితమైనదిగా పరిగణిస్తారు. అయితే, గింజలలో పీచుపదార్థం (ఫైబర్) ఎక్కువగా ఉండటం వల్ల, కొంతమంది మహిళలు ప్రారంభంలో కడుపు ఉబ్బరం లేదా గ్యాస్ వంటి తేలికపాటి జీర్ణ సంబంధిత అసౌకర్యాలను అనుభవించవచ్చు. ఇలాంటి ఇబ్బందులను తగ్గించుకోవడానికి, గింజల మోతాదును నెమ్మదిగా పెంచుతూ, శరీరానికి అలవాటు చేయడం మరియు రోజూ తగినంతగా నీరు త్రాగడం మంచిది.
సీడ్ సైక్లింగ్ ప్రధానంగా పిల్లలను కనే వయస్సులో (పునరుత్పత్తి సంవత్సరాలు) హార్మోన్ల సమతుల్యతకు మద్దతు ఇస్తుంది. అయినప్పటికీ, కొంతమంది మహిళలు ముట్లుడిగిపోయే ముందు దశలో (పెరిమెనోపాజ్) మరియు ముట్లుడిగిపోయిన ప్రారంభ దశలలో (ప్రారంభ మెనోపాజ్) కూడా దీనిని ప్రయోజనకరంగా భావించవచ్చు. గింజలలో ఉండే ఫైటోఈస్ట్రోజెన్లు (శరీరంలో ఈస్ట్రోజెన్ లాగా పనిచేసే మొక్కల నుండి వచ్చే పదార్థాలు) మరియు ముఖ్యమైన ఫ్యాటీ యాసిడ్లు వంటి పోషకాలు, మెనోపాజ్ సమయంలో వచ్చే మానసిక స్థితిలో మార్పులు, వేడి ఆవిర్లు (హాట్ ఫ్లషెస్), మరియు యోని పొడిబారడం వంటి కొన్ని లక్షణాలను తగ్గించడంలో సహాయపడవచ్చు. అయితే, ఈ విషయంలో ఇంకా ఎక్కువ శాస్త్రీయ పరిశోధన అవసరం. కాబట్టి, ఈ మార్పు సమయంలో మీ ఆరోగ్య పరిస్థితికి అనుగుణంగా సరైన మార్గదర్శకత్వం కోసం వైద్యుడిని సంప్రదించడం చాలా ముఖ్యం.
Fallopian tube obstruction, often caused by infections, endometriosis, or scar tissue, prevents the egg from meeting the sperm, which can lead to infertility. Blocked fallopian tubes hinder the natural fertilization process, making it difficult for a woman to conceive.
Diagnosis of fallopian tube obstruction is typically done through a hysterosalpingogram (HSG) or laparoscopy. Treatment options vary and may include minimally invasive procedures, surgery, or in vitro fertilization (IVF). Recovery time depends on the method of treatment, with some options allowing for quicker recovery than others.
Blocked fallopian tubes do not directly cause miscarriage but can increase the risk of ectopic pregnancy. The obstruction typically leads to infertility by preventing the egg and sperm from meeting, which can complicate the pregnancy process if conception does occur.
The success rates of IVF can vary widely and depend on various factors, such as the woman’s age, the underlying reason for infertility, and the clinic’s expertise. According to a report by the Centre for Disease Control and Prevention (CDC), the live childbirth rate per IVF cycle for women under 35 years old is around 35–40%. However, the success rates can be lower for older women or those with specific fertility issues.
There are several steps you can take to enhance your chances of a successful IVF cycle:
- Maintain a Healthy Lifestyle: Adopt a balanced diet, avoid processed food, exercise regularly, reduce stress, and avoid excessive alcohol intake and smoking.
- Manage Stress: Stress can negatively impact fertility, so practice stress-reducing techniques like meditation or yoga.
- Follow Your Doctor’s Instructions: Carefully follow your fertility specialist’s recommendations regarding medication, timing, and lifestyle.
- Consider Complementary Therapies: Some complementary therapies, like acupuncture, may improve IVF outcomes. Always consult your doctor before trying these approaches.
Pregnancy occurs when sperm fertilises an egg during the fertile window. This typically happens in the fallopian tubes after ovulation. The fertilised egg then travels to the uterus and implants itself, beginning the pregnancy journey. Successful conception usually requires intercourse during the five days leading up to ovulation or on the day of ovulation itself.
To increase the chances of conception, couples can time intercourse during the fertile window. Regular intercourse every 2-3 days throughout the cycle is also helpful. Additional steps include:
- Maintaining a healthy lifestyle and eating a balanced diet
- Tracking ovulation cycles accurately
- Taking prenatal vitamins with folic acid
- Avoiding alcohol and tobacco
- Managing stress effectively
Women under 35 are advised to seek medical help after one year of trying to conceive without success. Women over 35 should consult a healthcare provider after six months. Those over 40 or with known reproductive issues should seek help immediately.
Common myths include the belief that certain sexual positions can boost conception chances or that an orgasm is necessary for pregnancy. In reality, these are not supported by evidence. Also, birth control pills don’t cause long-term fertility problems, and moderate exercise does not hinder the ability to conceive.
Tracking ovulation helps identify the most fertile days in a cycle. Effective methods include:
- Using ovulation predictor kits
- Monitoring basal body temperature daily
- Observing changes in cervical mucus
- Using fertility tracking apps
- Keeping track of menstrual cycle dates
Maintaining a healthy lifestyle, tracking ovulation, managing stress, and seeking timely medical advice can all help improve the chances of conception. A balanced diet, regular moderate exercise, avoiding smoking and excessive alcohol, and maintaining a healthy weight are all important for fertility.
Couples can access support through fertility clinics, counseling services, government reproductive health programs, and assisted reproductive technologies like IVF and IUI. Many clinics also provide personalized treatment plans, emotional support, and expert guidance from fertility specialists.
No, infertility is not just a women’s issue. Men can be equally affected. Male infertility, such as low sperm count or poor sperm motility, can significantly impact a couple’s ability to conceive. Both partners should undergo fertility testing if pregnancy hasn’t occurred after one year of trying.
Common misconceptions in India include the belief that infertility is only a woman’s problem, that lifestyle habits don’t affect fertility, or that stress is the primary cause. In reality, both men and women can experience fertility issues, and factors like diet, exercise, smoking, and alcohol play a major role.
Yes, it is possible to get pregnant when overweight, but it may take longer due to hormonal imbalances and irregular ovulation. Maintaining a healthy weight can improve your chances of conceiving naturally and can also enhance the effectiveness of fertility treatments.
High-stress levels can disrupt hormones that regulate ovulation in women and testosterone in men, impacting the overall reproductive system. Chronic stress may lead to irregular cycles, poor sperm quality, and changes in libido, all of which can affect fertility outcomes.
BMI (Body Mass Index) plays a crucial role in fertility treatments. Both high and low BMI can affect hormone levels, ovulation, and the body’s readiness for pregnancy. Many fertility clinics recommend achieving a healthy BMI to increase the success rates of treatments such as IVF or IUI.
Regular exercise, a balanced and nutritious diet, managing stress, and avoiding unhealthy habits like excessive alcohol intake and smoking can improve fertility. These lifestyle changes support hormonal balance, ovulation, and overall reproductive health, thereby enhancing the chances of conception.
The AMH test is a simple blood test for women that measures the level of Anti-Müllerian Hormone. This hormone level helps to indicate the number of eggs a woman has left, which is known as her ovarian reserve.
A major advantage of the AMH test is that it can be done at any time during your menstrual cycle. The hormone levels remain stable, so you don’t need to schedule it for a specific day.
In women, the Anti-Müllerian Hormone is produced by the small developing egg sacs (follicles) in the ovaries. Its level in the blood reflects the size of the remaining egg supply.
An AMH test is often recommended if you have been trying to conceive without success, are considering fertility treatments like IVF, or if you want to gain insight into your current fertility potential for future planning.
A low AMH level (typically below 1.0 ng/mL) indicates a reduced ovarian reserve, not necessarily infertility. While it may be more challenging to conceive, pregnancy is still possible. It’s a marker of egg quantity, not quality.
Yes, you can get pregnant with high AMH. High levels often indicate a large number of eggs. However, very high levels can sometimes be associated with conditions like Polycystic Ovary Syndrome (PCOS), which may affect ovulation.
No, fasting is not required for an AMH test. You can eat and drink normally before the test.
Yes, the AMH test can be done at any time, including during your period. The results are not affected by your menstrual cycle.
You can get your AMH tested by visiting a fertility clinic or a gynecologist. They will order a simple blood test, where a small sample of blood is drawn from a vein in your arm.
Yes, it can be very worthwhile. The test provides valuable information about your ovarian reserve, which can help you and your doctor make informed decisions about your fertility, family planning, and potential treatments.
Normal AMH levels vary significantly by age. A fertility specialist can best interpret your results, but generally, a level between 1.0 ng/mL and 3.0 ng/mL is considered a normal and healthy range for women of reproductive age.
No, the test is not painful at all. The analysis is performed on a semen sample in a lab. The collection process itself is done privately through masturbation and is non-invasive, so there is no physical pain involved.
Yes, age can affect semen quality. While men continue to produce sperm throughout their lives, the quality, including sperm count, movement, and DNA integrity, can gradually decline, particularly after the age of 40.
Abstaining from ejaculation for 2 to 5 days is important because it allows the sperm count in the semen to build up to a stable level. This ensures the sample is concentrated enough for an accurate and reliable analysis of your sperm health.
Yes, infections in the male reproductive tract can negatively affect semen analysis results. They can reduce sperm count and motility (movement) and even cause inflammation that damages sperm. Treating the underlying infection often improves these parameters.
Absolutely. Positive lifestyle changes can significantly improve sperm health. This includes eating a balanced diet, exercising regularly, maintaining a healthy weight, quitting smoking, reducing alcohol consumption, and managing stress.
వీటి మధ్య ఉన్న ఏకైక తేడా, ల్యాబ్లో ఫలదీకరణం ఎలా జరుగుతుంది అనే దానిలోనే ఉంటుంది.
- సాధారణ IVF: వీర్యకణాలు, అండాలను ఒక డిష్లో కలిపి ఉంచుతారు, దీనివల్ల ఫలదీకరణం సహజంగా జరుగుతుంది.
ICSI: పిండ శాస్త్రవేత్త (Embryologist) ఎంపిక చేసిన ఒకే ఒక్క వీర్యకణాన్ని నేరుగా అండంలోకి ఇంజెక్ట్ చేస్తారు.
ICSI చాలా సురక్షితమైన ప్రక్రియ. దీని వల్ల ప్రమాదాలు సాధారణ IVF తో సమానంగా ఉంటాయి మరియు భారతదేశంలోని అన్ని క్లినిక్లలో ప్రామాణిక పద్ధతులను ఉపయోగించి వీటిని నిర్వహిస్తారు:
- ఒవేరియన్ హైపర్స్టిమ్యులేషన్ సిండ్రోమ్ (OHSS): ఇది ఫెర్టిలిటీ మందుల వల్ల కలిగే ఒక అరుదైన ప్రతిచర్య.
- కవలలు పుట్టడం: ఒకటి కంటే ఎక్కువ పిండాలను బదిలీ చేస్తే కవలలు పుట్టే అవకాశం ఎక్కువగా ఉంటుంది.
- అండం దెబ్బతినడం: ఇంజెక్షన్ సమయంలో అండం దెబ్బతినే ప్రమాదం చాలా చాలా తక్కువ (1% కన్నా తక్కువ). నైపుణ్యం కలిగిన పిండ శాస్త్రవేత్తల చేతిలో ఇది దాదాపు జరగదు.
లేదు, అసలైన ICSI ప్రక్రియ ల్యాబ్లో జరుగుతుంది కాబట్టి, అది నొప్పిగా ఉండదు. చికిత్సా సైకిల్ సమయంలో, రోగికి ఈ కింది అనుభవాలు ఉంటాయి:
- రోజువారీ హార్మోన్ ఇంజెక్షన్ల వల్ల తేలికపాటి అసౌకర్యం.
- అండం సేకరణ ప్రక్రియ అనస్థీషియా (మత్తు మందు) ఇచ్చి చేస్తారు కాబట్టి అప్పుడు నొప్పి ఉండదు.
- అండం సేకరించిన తర్వాత ఒకటి లేదా రెండు రోజులు నెలసరి నొప్పిలాగా, తట్టుకోగలిగే కడుపునొప్పి ఉండవచ్చు.
భారతదేశంలో, అలాగే ప్రపంచవ్యాప్తంగా, ఫలదీకరణం సాధించడానికి ICSI ఒక అద్భుతమైన పద్ధతి, కానీ ఇది గర్భధారణకు హామీ ఇవ్వదు. వైఫల్యానికి ప్రాథమిక కారణాలు జీవసంబంధమైనవి. మరియు మా రోగ నిర్ధారణ ప్రక్రియలో మేము వీటిపైనే ప్రధానంగా దృష్టి పెడతాము:
- అండం నాణ్యత సరిగా లేకపోవడం: ఇది అత్యంత సాధారణ కారణం, తరచుగా మహిళ వయసుతో ముడిపడి ఉంటుంది. ఫలదీకరణం చెందిన అండం ఆరోగ్యకరమైన పిండంగా మారే శక్తిని కలిగి ఉండకపోవచ్చు.
- పిండం నాణ్యత సరిగా లేకపోవడం: అండం లేదా వీర్యకణంలో అంతర్లీనంగా ఉండే జన్యుపరమైన సమస్యల వల్ల, పిండం కొన్ని రోజుల తర్వాత అభివృద్ధి చెందడం ఆగిపోవచ్చు.
ఇంప్లాంటేషన్ వైఫల్యం: ఆరోగ్యకరమైన పిండం కూడా, గర్భాశయ లోపలి పొరలో సమస్యలు లేదా ఇతర కారణాల వల్ల దానికి అతుక్కోవడంలో విఫలమవవచ్చు.
PICSI యొక్క ప్రధాన ప్రయోజనం, అత్యుత్తమ వీర్యకణాన్ని ఎంచుకునే దాని అధునాతన పద్ధతి. ఈ టెక్నిక్, పరిపక్వ మరియు అపరిపక్వ వీర్యకణాల మధ్య తేడాను గుర్తించడానికి పిండ శాస్త్రవేత్తలకు (Embryologists) వీలు కల్పిస్తుంది. పరిపక్వ వీర్యకణాలు పూర్తిగా అభివృద్ధి చెంది ఉంటాయి మరియు వాటిలో DNA దెబ్బతినే లేదా క్రోమోజోముల సంఖ్యలో లోపాలు ఉండే అవకాశం తక్కువ. అందువల్ల చికిత్సలో వాడటానికి ఇవి ఉత్తమమైనవి.
PICSI, మానవ శరీరంలో సహజంగా ఉండే హైలురోనిక్ యాసిడ్ (HA) అనే పదార్థానికి వీర్యకణాలను గురిచేయడం ద్వారా పనిచేస్తుంది. ఈ ప్రక్రియలో, HAకు అతుక్కోగల సామర్థ్యం ఉన్న వీర్యకణాలను గుర్తిస్తారు, మరియు వాటినే సంతాన చికిత్సలలో ఉపయోగించడానికి ఎంపిక చేస్తారు.
సాధారణ ICSI తో PICSI ని పోలుస్తూ చేసిన పరిశోధనలలో, PICSI తో గణనీయంగా అధిక ఫలదీకరణ రేట్లు సాధించవచ్చని తేలింది. అంతేకాకుండా, దీని ద్వారా ట్రాన్స్ఫర్ చేయడానికి అనువైన పిండాల సంఖ్య పెరగడంతో పాటు, అధిక నాణ్యత గల పిండాలు ఎక్కువగా ఉత్పత్తి అవుతాయని కూడా నిరూపించబడింది.
అవును, PICSI పిండం నాణ్యతను గణనీయంగా మెరుగుపరుస్తుంది. పిండం గర్భాశయానికి అతుక్కునే (ఇంప్లాంటేషన్) సామర్థ్యం పెరగడం మరియు అన్యూప్లాయిడీస్ (క్రోమోజోముల సంఖ్యలో ఉండే అసాధారణతలు) ప్రమాదం తగ్గడమే దీనికి నిదర్శనం.
Booking your AMH test at Ferty9 in Hyderabad is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Hyderabad directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Hyderabad, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Hyderabad center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Hyderabad center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Hyderabad is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Kukatpally is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Kukatpally directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Kukatpally, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Kukatpally center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Kukatpally center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Kukatpally is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Lb Nagar is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Lb Nagar directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Lb Nagar, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Lb Nagar center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Lb Nagar center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Lb Nagar is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Secunderabad is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Secunderabad directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Secunderabad, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Secunderabad center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Secunderabad center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Secunderabad is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Banjara Hills is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Banjara Hills directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Banjara Hills, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Banjara Hills center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Banjara Hills center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Banjara Hills is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Visakhapatnam is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Visakhapatnam directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Visakhapatnam, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Visakhapatnam center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Visakhapatnam center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Visakhapatnam is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Vijayawada is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Vijayawada directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Vijayawada, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Vijayawada center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Vijayawada center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Vijayawada is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Karimnagar is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Karimnagar directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Karimnagar, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Karimnagar center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Karimnagar center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Karimnagar is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Warangal is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Warangal directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Warangal, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Warangal center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Warangal center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Warangal is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Rajahmundry is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Rajahmundry directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Rajahmundry, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Rajahmundry center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Rajahmundry center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Rajahmundry is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Tirupati is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Tirupati directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Tirupati, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Tirupati center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Tirupati center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Tirupati is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Booking your AMH test at Ferty9 in Kurnool is simple and convenient. You can:
- Call Us Directly: Speak with our front desk to schedule an appointment at a time that works for you.
- Walk-In: You can visit our Ferty9 Fertility Center in Kurnool directly. As no prior preparation like fasting is needed, you can get the test done whenever you are ready.
- Online Inquiry: Visit our Ferty9 website and fill out the appointment booking form, and our local team will get in touch with you.
Our center is centrally located in Kurnool, making it easily accessible for you.
At Ferty9, we are committed to providing affordable healthcare. The cost of an AMH test at our Kurnool center is very competitive. While the exact price can vary slightly based on any ongoing offers or packages, we ensure it is one of the most budget-friendly options available. For the precise and current cost, please call our Kurnool center directly.
We understand that waiting for test results can be anxious. That’s why Ferty9 Kurnool is equipped with an advanced in-house laboratory that allows us to process tests efficiently. We prioritize a quick turnaround and provide reports for the AMH test by the next day of receiving blood sample. You can get your blood sample taken in the morning and receive your report by the evening.
The AMH test is a very safe blood test that assesses key aspects of your fertility. It isn’t risky, but rather it evaluates your reproductive potential by measuring three main things:
- Ovarian Reserve: It gives a reliable estimate of your current egg count.
- Fertility Treatment Response: It helps predict how your body might respond to treatments like IVF.
Hormonal Indicators: Unusually high levels can be an indicator for conditions like PCOS.
Yes, absolutely. Andrologists are trained surgeons who specialize in the male reproductive system. They routinely perform procedures like varicocele repair, vasectomy reversals, and advanced sperm retrieval techniques (TESA, PESA, Micro-TESE) to help treat male infertility.
Yes. Diagnosing testicular abnormalities is a core part of an andrologist’s expertise. Using physical examinations and scrotal ultrasounds, they can identify conditions like varicoceles, cysts, blockages, and other issues that may affect fertility or health.
Yes. Andrologists are experts in managing the male hormonal system. They frequently diagnose and treat conditions like low testosterone, which can affect libido, energy levels, and sperm production, often using medication and lifestyle guidance.
It depends on the cause. If the zero sperm count is due to a blockage (obstructive azoospermia), surgery can often fix the issue and restore sperm to the ejaculate. If its a production problem, a “cure” may not be possible, but andrologists can often still retrieve sperm directly from the testicles using advanced procedures, making fatherhood achievable through IVF/ICSI.
Yes. Andrologists can diagnose and treat various ejaculatory disorders, including premature ejaculation, delayed ejaculation, and retrograde ejaculation (where ejaculate enters the bladder). Treatment often involves medication, specific techniques, or therapy.
Yes, this is a very important role. Before a man undergoes cancer treatments like chemotherapy or radiation that can harm fertility, an andrologist can counsel him on fertility preservation options, primarily through sperm freezing (cryopreservation), to preserve his ability to have children in the future.
The AMH test cost in India is generally affordable. For the most accurate and current pricing, please contact Ferty9 directly.
Insurance coverage for fertility tests is not available at Ferty9.
If you are searching for “where to get an AMH test,” look no further. You can easily book your test at any of our Ferty9 locations.
Ferty9’s ovulation calculator provides estimates with accuracy varying based on cycle regularity. Our calculations are based on research showing traditional calendar methods achieve 70-89% accuracy for certain prediction aspects, though individual variation affects reliability. Our calculator works best for women with regular cycles and should be combined with other tracking methods for improved precision, which our fertility specialists often recommend.
Ferty9’s ovulation calculator can still provide value for irregular cycles, but with modified expectations that our fertility team discusses with patients. Use our calculator as a general guide rather than a precise predictor, track multiple cycles to identify subtle patterns, and consider consulting our specialists about combining it with other methods like ovulation predictor kits or basal body temperature tracking for better accuracy.
To use our ovulation calculator effectively, you need the first day of your last menstrual period and your average cycle length. Calculate cycle length by counting days from the first day of one period to the first day of the next period, tracking at least three cycles for accuracy. Our user-friendly interface also allows input of additional information that can improve predictions.
Ferty9’s ovulation calculator identifies a fertile window spanning approximately six days: the five days before ovulation plus the day of ovulation. This accounts for sperm survival of up to five days in the reproductive tract and the 12-24 hour egg viability period. Our fertility specialists emphasize that the highest conception probability occurs during the final three days of this window.
Our fertility experts at Ferty9 strongly advise that ovulation calculators should never be used as the sole method of contraception. While our calculator can provide general cycle awareness, prediction limitations make it unreliable for preventing pregnancy. We recommend professional contraceptive methods or properly trained fertility awareness methods for pregnancy prevention.
Ferty9’s medical team identifies common ovulation signs including clear, stretchy cervical mucus resembling egg whites, mild one-sided abdominal cramping (mittelschmerz), breast tenderness, slight temperature rise, increased libido, and sometimes light spotting. Our fertility specialists note that not all women experience these symptoms, and they can vary between cycles.
Our ovulation calculator accommodates various cycle lengths, typically from 21-35 days, which covers normal healthy variation recognized by our medical team. The calculator adjusts predictions based on your specific cycle length, though women with cycles outside this range may need evaluation by our fertility specialists for underlying health conditions.
Our fertility experts recommend beginning to use our ovulation calculator when you start actively trying to conceive or want to understand your cycle better. Track for at least 2-3 cycles to establish patterns and improve prediction accuracy. Starting early allows you to learn your body’s signals and optimize timing, which aligns with Ferty9’s comprehensive fertility approach.
Yes, our fertility specialists at Ferty9 recognize that stress can significantly impact ovulation timing by disrupting hormonal balance and potentially delaying or preventing ovulation. During high-stress periods, our calculator predictions may be less reliable. We often recommend stress management techniques and noting stressful events when tracking cycles.
Ferty9’s ovulation calculator is specifically designed based on our clinical expertise in fertility medicine, providing medically accurate predictions integrated with our other fertility services. Unlike basic calculators, ours offers educational content, connects with our period and pregnancy calculators, and provides pathways to consultation with our fertility specialists when needed.
Our calculator provides predictions for the current cycle and several months ahead based on your average cycle length. However, our fertility team notes that accuracy decreases for longer-term predictions due to natural cycle variations. We recommend focusing on current and next-cycle predictions for planning purposes.
Our medical team explains that hormonal birth control methods suppress natural ovulation, making traditional ovulation calculators irrelevant since they prevent the hormonal cycles our calculator predicts. If using non-hormonal methods like copper IUDs, natural cycles continue and our calculator remains useful for cycle awareness.
While our calculator cannot diagnose fertility issues, consistent tracking may reveal patterns suggesting problems such as very irregular cycles, absent ovulation signs, or cycles outside normal ranges. These observations can prompt appropriate medical evaluation with our fertility specialists, who can provide comprehensive assessment and treatment options.
Our fertility experts recommend tracking predicted dates against actual ovulation symptoms like cervical mucus changes, temperature rises, or positive ovulation tests. Over several cycles, you’ll notice if our predictions align with physical signs. Consistent discrepancies may indicate need for adjusted cycle length input or consultation with our medical team.
Absence of noticeable ovulation symptoms doesn’t necessarily indicate problems, as our fertility specialists note that not all women experience obvious signs. However, if you consistently lack signs and have difficulty conceiving, consider using ovulation predictor kits or consulting our healthcare providers to confirm ovulation is occurring and explore additional support options available at Ferty9.
Gynecology is the branch of medicine focused on the health of a woman’s reproductive system. This includes the uterus (womb), ovaries, fallopian tubes, and breasts. It covers all aspects of a woman’s reproductive health from her teenage years through menopause.
A gynecologist is a doctor who specializes in women’s reproductive health. Their work includes diagnosing and treating issues related to periods, infections, PCOD/PCOS, uterine fibroids, and fertility. They also perform important health screenings like Pap tests for cervical cancer and provide advice on family planning.
While many doctors are both (known as OB-GYNs), their roles are different:
- A Gynecologist cares for a woman’s general reproductive health when she is not pregnant.
- An Obstetrician specifically cares for a woman during pregnancy, childbirth, and the period just after delivery.
It is generally recommended for a girl to have her first visit with a gynecologist between the ages of 13 and 15. This first visit is usually just a friendly conversation to discuss periods and general health. All adult women should have regular check-ups as advised by their doctor.
Gynecologists perform various tests depending on the need of patient health needs. Common tests include a physical pelvic exam, a Pap smear to screen for cervical cancer, ultrasound scans to get images of the uterus and ovaries, and blood tests to check hormone levels.
The consultation fee for a gynecologist in Hyderabad typically ranges from ₹600 to ₹1500. The cost can vary based on the doctors experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Hyderabad location to schedule a visit.
To find the best gynecologist in Kukatpally, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Kukatpally typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Kukatpally location to schedule a visit.
To find the best gynecologist in LB Nagar, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in LB Nagar typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred LB Nagar location to schedule a visit.
To find the best gynecologist in Secunderabad, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Secunderabad typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Secunderabad location to schedule a visit.
To find the best gynecologist in Banjara Hills, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Banjara Hills typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Banjara Hills location to schedule a visit.
To find the best gynecologist in Visakhapatnam, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Visakhapatnam typically ranges from ₹600 to ₹1500. The cost can vary based on the doctors experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Visakhapatnam location to schedule a visit.
To find the best gynecologist in Vijayawada, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Vijayawada typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Vijayawada location to schedule a visit.
To find the best gynecologist in Karimnagar, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Karimnagar typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Karimnagar location to schedule a visit.
To find the best gynecologist in Warangal, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Warangal typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Warangal location to schedule a visit.
To find the best gynecologist in Rajahmundry, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Rajahmundry typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Rajahmundry location to schedule a visit.
To find the best gynecologist in Tirupati, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Tirupati typically ranges from ₹600 to ₹1500. The cost can vary based on the doctor’s experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Tirupati location to schedule a visit.
To find the best gynecologist in Kurnool, you can start by asking for recommendations from family or friends. Reading online patient reviews and checking the websites of reputable hospitals and specialized clinics like Ferty9 Fertility Center can also help you find a doctor with expertise in the area you need.
The consultation fee for a gynecologist in Kurnool typically ranges from ₹600 to ₹1500. The cost can vary based on the doctors experience and the location of the clinic. Please note that this fee is for the consultation only, and any tests or procedures will be charged separately.
At Ferty9, our gynecologists are also expert fertility specialists. Their services are focused on helping individuals and couples achieve pregnancy. This includes:
- Diagnosing the root cause of infertility.
- Managing conditions like PCOD/PCOS and endometriosis to improve fertility.
- Providing treatments like IUI, IVF, and ICSI.
- Offering expert counselling and support before and during pregnancy.
Booking a consultation is simple. You can search online for “gynecologist near me” and read reviews. For a specialized centre like Ferty9, you can visit our website to fill out an appointment form or directly call our clinic in your preferred Kurnool location to schedule a visit.
అవును. క్రమం తప్పిన సైకిల్స్ ఓవులేషన్ను ప్రభావితం చేస్తాయి, దీనివల్ల మీరు ఎప్పుడు అత్యంత ఫలవంతంగా ఉన్నారో గుర్తించడం కష్టం అవుతుంది. అంతేకాకుండా, మీకు క్రమం తప్పిన నెలసరి ఉంటే, మీరు క్రమం తప్పకుండా అండాన్ని విడుదల చేయరు (ఓవులేట్) కాబట్టి గర్భం దాల్చడం మరింత కష్టతరం కావచ్చు.
ఓవులేషన్కు సహాయపడే ఆహారాలలో బీన్స్, కాయధాన్యాలు (పప్పులు), సముద్రపు ఆహారం, ఆకుకూరలు, గుడ్లు, పండ్లు మరియు కూరగాయలు, నట్స్ మరియు విత్తనాలు, మరియు సంపూర్ణ ధాన్యాలు ఉన్నాయి.
కాదు. డాక్టర్ను సంప్రదించకుండా ఓవులేషన్ను పెంచడానికి హెర్బల్ సప్లిమెంట్లను తీసుకోవడం సురక్షితం కాదు. కొన్ని మూలికలు ఇతర మందులతో లేదా సప్లిమెంట్లతో ప్రతిచర్య జరిపి, అనుకోని హార్మోన్ల పరిణామాలకు కారణం కావచ్చు.
ఒక సంవత్సరం కంటే ఎక్కువ కాలం ప్రయత్నించిన తర్వాత కూడా గర్భం దాల్చడంలో విజయం సాధించకపోతే, ఓవులేషన్ సమస్యల గురించి డాక్టర్ను సంప్రదించాల్సిన సమయం కావచ్చు. క్రమం తప్పిన సైకిల్స్, నెలసరి రాకపోవడం, లేదా హార్మోన్ల అసాధారణతల లక్షణాలు వంటివి వైద్య నిపుణులను సంప్రదించడానికి గల కారణాలు.
సంతాన సామర్థ్యాన్ని పెంచుకోవడానికి ప్రతిరోజూ 1,000 నుండి 2,000 mg ఒమేగా-3 ఫ్యాటీ యాసిడ్లను తీసుకోవాలని సిఫార్సు చేయబడింది. ఒమేగా-3 ఫ్యాటీ యాసిడ్లు శుక్రకణాలు మరియు అండాలతో సహా అన్ని కణాల అభివృద్ధి మరియు నిర్మాణంలో కీలక పాత్ర పోషిస్తాయి, ఇవి విజయవంతమైన గర్భధారణకు చాలా అవసరం.
అవును. ఒమేగా-3 సప్లిమెంట్లు సైక్లోస్పోరిన్లు, యాంటీకోయాగ్యులెంట్లు (రక్తాన్ని పలచబరిచే మందులు), మరియు రక్తపోటును తగ్గించే మందులతో సహా కొన్ని మందులతో ప్రతిచర్య జరపవచ్చు. వాటిని తీసుకోవడం ప్రారంభించే ముందు, ఒమేగా-3లకు మరియు ఇతర మందులు లేదా సప్లిమెంట్లకు మధ్య సంభావ్య ప్రతిచర్యల గురించి మీ ఆరోగ్య నిపుణులను (డాక్టర్ను) సంప్రదించడం చాలా అవసరం.
అవును. సంతాన సాఫల్య చికిత్సలు పొందుతున్న వ్యక్తులకు ఒమేగా-3 డైటరీ సప్లిమెంట్లు ప్రయోజనకరంగా ఉంటాయి. ఇవి ఫలదీకరణ రేట్లను మెరుగుపరుస్తాయని మరియు పిండం నాణ్యతను పెంచుతాయని నిరూపించబడింది, తద్వారా గర్భధారణ అవకాశాలను పెంచుతాయి, ముఖ్యంగా IVF చికిత్సలు పొందుతున్న మహిళలకు.
అవును. గర్భస్రావం లేదా విఫలమైన IVF సైకిల్ తర్వాత ఒమేగా-3లు సంతానోత్పత్తికి మద్దతు ఇవ్వగలవు. అవి కణ గోడల సమగ్రతను కాపాడటం, శక్తిని అందించడం, మరియు నెలలు నిండని ప్రసవాలు, గర్భస్రావాలు, మరియు మృత శిశు జననాలు వంటి సమస్యల అవకాశాలను తగ్గించడం ద్వారా సహాయపడతాయి. మీరు కోలుకునే ప్రణాళికలో ఒమేగా-3లను చేర్చుకోవడం వలన పునరుత్పత్తి ఆరోగ్యాన్ని మెరుగుపరచవచ్చు మరియు భవిష్యత్ సంతాన ఫలితాలను మెరుగుపరచవచ్చు.
చాలా మంది రోగులు ప్రక్రియ జరిగిన తర్వాత 24-48 గంటల పాటు కడుపు నొప్పిని అనుభవిస్తారు. కొందరిలో తేలికపాటి అసౌకర్యం 5 రోజుల వరకు ఉండవచ్చు, అయితే ఇది వ్యక్తులను బట్టి మారుతుంది. నొప్పి తీవ్రత సాధారణంగా మొదటి కొన్ని గంటలలో గరిష్ట స్థాయికి చేరుకుని, క్రమంగా తగ్గుతుంది.
లేదు. కడుపు నొప్పి రావడం IUI ప్రక్రియ యొక్క విజయాన్ని గానీ, వైఫల్యాన్ని గానీ సూచించదు. ఈ అనుభూతులు కేవలం చికిత్సకు శరీరం యొక్క సహజ ప్రతిస్పందనను చూపుతాయి. కొన్ని విజయవంతమైన గర్భధారణలలో కడుపు నొప్పి ఉంటుంది, మరికొన్నింటిలో ఉండదు.
IUI తర్వాత అసౌకర్యాన్ని తగ్గించుకోవడానికి డాక్టర్లు అనేక ప్రభావవంతమైన పద్ధతులను సిఫార్సు చేస్తారు:
- హీటింగ్ ప్యాడ్ ఉపయోగించడం
- డాక్టర్ సూచించిన నొప్పి నివారణ మందులు తీసుకోవడం
- పుష్కలంగా నీరు త్రాగి హైడ్రేటెడ్గా ఉండటం
- తగినంత విశ్రాంతి తీసుకోవడం
- తేలికపాటి స్ట్రెచింగ్ వ్యాయామాలు చేయడం
అవును. శారీరక శ్రమ స్థాయిలు మరియు ఒత్తిడి కడుపు నొప్పి తీవ్రతను ప్రభావితం చేయగలవు. తేలికపాటి కార్యకలాపాలు సాధారణంగా ప్రయోజనకరంగా ఉంటాయి, కానీ అతిగా శ్రమించడం అసౌకర్యాన్ని పెంచవచ్చు. తగినంత విశ్రాంతితో కూడిన సమతుల్య జీవనశైలిని పాటించడం లక్షణాలను సమర్థవంతంగా నిర్వహించడంలో సహాయపడుతుంది.
ఫెర్టిలిటీ మందులు కడుపు నొప్పి అనుభూతిని తీవ్రతరం చేయవచ్చు. వివిధ మందులు రోగులను విభిన్నంగా ప్రభావితం చేస్తాయి, ముఖ్యంగా హార్మోన్ల ఆధారిత చికిత్సలు మరింత గమనించదగిన ప్రభావాలను కలిగించవచ్చు. శరీరం చికిత్సకు సర్దుబాటు చేసుకున్న కొద్దీ మందులకు సంబంధించిన ఈ లక్షణాలు సాధారణంగా తగ్గిపోతాయి.
తీవ్రమైన నొప్పికి తక్షణ వైద్య సహాయం అవసరం, ముఖ్యంగా జ్వరం, అధిక రక్తస్రావం, లేదా తీవ్రమైన పొత్తికడుపు ఒత్తిడి వంటి లక్షణాలతో కూడి ఉన్నప్పుడు. నొప్పి తీవ్రంగా మారినా లేదా ఒక వారం దాటినా కొనసాగితే, రోగులు తమ ఫెర్టిలిటీ డాక్టర్లను సంప్రదించాలి.
అవును గర్భాశయ క్షయ (TB) ముఖ్యంగా ప్రారంభ దశలలో ఎటువంటి లక్షణాలు లేకుండా ఉండవచ్చు. గర్భాశయ క్షయ ఉన్న చాలా మంది మహిళలలో కనిపించే లక్షణాలు ఉండకపోవచ్చు లేదా వారి లక్షణాలు చాలా తేలికపాటివిగా ఉండవచ్చు, దీనివల్ల పూర్తిస్థాయి వైద్య పరీక్షలు లేకుండా ఈ పరిస్థితిని నిర్ధారించడం కష్టం.
గర్భాశయ క్షయ (TB) నేరుగా ఒకరి నుండి మరొకరికి అంటుకునే వ్యాధి కాదు. గర్భాశయ క్షయ అనేది, శరీరంలోని ఇతర భాగాల నుండి, ముఖ్యంగా ఊపిరితిత్తుల నుండి, క్షయ బాక్టీరియా రక్త ప్రవాహం లేదా శోషరస వ్యవస్థ ద్వారా పునరుత్పత్తి అవయవాలకు చేరినప్పుడు సంభవిస్తుంది. దీనిని బట్టి గర్భాశయ క్షయ అనేది ఇతరులతో లైంగిక లేదా సన్నిహిత శారీరక సంబంధం ద్వారా కాకుండా, శరీరం లోపల అంతర్గతంగా వ్యాపించడం వల్ల వస్తుందని తెలుస్తుంది.
గర్భాశయ TBకి చికిత్స ఎంతకాలం పడుతుంది?గర్భాశయ క్షయ చికిత్సకు సాధారణంగా దీర్ఘకాలిక యాంటీ-ట్యూబర్క్యులోసిస్ మందుల కోర్సు ఉంటుంది. వ్యాధి యొక్క తీవ్రత మరియు చికిత్సకు వ్యక్తిగత స్పందనను బట్టి ఇది కొన్ని నెలల నుండి ఒక సంవత్సరం వరకు పట్టవచ్చు.
అవును. గర్భాశయ క్షయ వల్ల కలిగే సంతానలేమికి ఇన్ విట్రో ఫెర్టిలైజేషన్ (IVF) సహాయపడుతుంది. ముఖ్యంగా ఫెలోపియన్ ట్యూబ్స్ వంటి పునరుత్పత్తి అవయవాలకు విస్తృతమైన నష్టం జరిగినప్పుడు మరియు దానిని మందులు లేదా శస్త్రచికిత్సతో సులభంగా పరిష్కరించలేనప్పుడు IVF ఒక మంచి మార్గం.
సంతాన సామర్థ్యానికి అనుకూలమైన ఆహారంలో యాంటీఆక్సిడెంట్లు మరియు అవసరమైన పోషకాలు అధికంగా ఉండే సంపూర్ణ ఆహారాలపై దృష్టి పెట్టాలి. అత్యంత ప్రయోజనకరమైన ఆహారాలలో ఆకుకూరలు, నట్స్, విత్తనాలు, కొవ్వు అధికంగా ఉండే చేపలు, మరియు సంపూర్ణ ధాన్యాలు ఉన్నాయి. ఈ ఆహారాలు ఫోలేట్, ఒమేగా-3 ఫ్యాటీ యాసిడ్లు, మరియు జింక్ వంటి పునరుత్పత్తి ఆరోగ్యానికి మద్దతు ఇచ్చే ముఖ్యమైన పోషకాలను అందిస్తాయి.
దీర్ఘకాలిక ఒత్తిడి హార్మోన్ల సమతుల్యతకు మరియు నెలసరి క్రమానికి ఆటంకం కలిగించడం ద్వారా సంతాన సామర్థ్యంపై గణనీయంగా ప్రభావం చూపుతుందని పరిశోధనలు నిర్ధారిస్తున్నాయి. అధిక ఒత్తిడి ఉన్నవారు, తక్కువ ఒత్తిడి ఉన్నవారితో పోలిస్తే గర్భం దాల్చడానికి 29% ఎక్కువ సమయం పట్టవచ్చని అధ్యయనాలు చెబుతున్నాయి. సరైన సంతాన సామర్థ్యాన్ని కాపాడుకోవడానికి క్రమం తప్పని ఒత్తిడి నిర్వహణ చాలా ముఖ్యం.
చాలా మంది స్థిరంగా సంతాన సామర్థ్యాన్ని పెంచే ఆహారాన్ని అనుసరించిన మూడు నుండి నాలుగు నెలలలోపు వారి పునరుత్పత్తి ఆరోగ్యంలో మెరుగుదలలను గమనించడం ప్రారంభిస్తారు. ఈ సమయం అండం మరియు శుక్రకణాల అభివృద్ధి యొక్క పూర్తి చక్రానికి సరిపోతుంది, అందువల్ల అర్థవంతమైన మార్పులు చూడటానికి ఇది కనీస కాలం.
సంతాన సామర్థ్యాన్ని పెంచే మూలికలు నిపుణుల మార్గదర్శకత్వంలో సరిగ్గా ఉపయోగించినప్పుడు సురక్షితంగా ఉండవచ్చు. అయితే, అన్ని మూలికలు అందరికీ సరిపోవు; కొన్ని మందులతో ప్రతిచర్య జరపవచ్చు. ఏదైనా హెర్బల్ సప్లిమెంట్లను ప్రారంభించే ముందు, ముఖ్యంగా మీరు సంతాన సాఫల్య చికిత్సలు తీసుకుంటుంటే, ఎల్లప్పుడూ నిపుణులైన వైద్యుడిని లేదా ఆయుర్వేద డాక్టర్ను సంప్రదించండి.
సంతాన సామర్థ్యాన్ని పెంచడానికి అవసరమైన విటమిన్లు:
- ఫోలిక్ యాసిడ్ (రోజుకు 400–800 mcg)
- విటమిన్ D3 (2,000–3,000 IU)
- CoQ10 (200–600 mg)
- ఒమేగా-3 ఫ్యాటీ యాసిడ్లు (1,000–2,000 mg)
ఈ పోషకాలు అండం నాణ్యత, హార్మోన్ల సమతుల్యత, మరియు మొత్తం పునరుత్పత్తి ఆరోగ్యానికి మద్దతు ఇస్తాయి.
IVF సైకిల్ ప్రారంభించడానికి కనీసం మూడు నెలల ముందు మద్యం సేవించడం మానేయాలని డాక్టర్లు సాధారణంగా సిఫార్సు చేస్తారు. ఈ కాలం శరీరం కోలుకోవడానికి మరియు విజయానికి మెరుగైన అవకాశం కోసం సంతాన సామర్థ్య స్థాయిలను ఉత్తమంగా ఉంచుకోవడానికి అనుమతిస్తుంది.
అవును, అప్పుడప్పుడు లేదా మోస్తరుగా మద్యం సేవించడం కూడా IVF ఫలితాలను ప్రభావితం చేసే అవకాశం ఉంది. సంబంధిత ప్రమాదాలను తగ్గించడానికి మరియు విజయవంతమైన ఫలదీకరణ మరియు ఇంప్లాంటేషన్ అవకాశాలను గరిష్టంగా పెంచుకోవడానికి IVF ప్రక్రియలో మద్యానికి పూర్తిగా దూరంగా ఉండటం ఉత్తమం.
అవును, మద్యం సేవించడం విజయవంతమైన IVF సైకిల్కు అవసరమైన సున్నితమైన హార్మోన్ల సమతుల్యతకు ఆటంకం కలిగిస్తుంది. ఇది అండం విడుదల (ఓవులేషన్) సమయం లేదా మందులకు శరీరం స్పందించే తీరును ప్రభావితం చేయవచ్చు, కాబట్టి సూచించిన షెడ్యూల్ను అనుసరించడం మరియు ప్రక్రియ అంతటా మద్యానికి దూరంగా ఉండటం చాలా అవసరం.
మీరు నేరుగా తాగడం అంత ప్రత్యక్ష ప్రభావం చూపనప్పటికీ, ఇతరులు ఎక్కువగా మద్యం సేవిస్తున్న వాతావరణాలకు దూరంగా ఉండటం మంచిది. IVF చికిత్స సమయంలో ఒత్తిడి మరియు సంభావ్య విష పదార్థాలను తగ్గించడం మెరుగైన ఫలితాలకు దోహదం చేస్తుంది.
మద్యం సేవించడం హార్మోన్ల స్థాయిలను మార్చడం మరియు పిండాన్ని స్వీకరించే గర్భాశయ సామర్థ్యాన్ని తగ్గించడం ద్వారా ఫ్రోజెన్ ఎంబ్రియో ట్రాన్స్ఫర్స్ (FET) విజయ రేట్లను ప్రతికూలంగా ప్రభావితం చేస్తుంది. విజయవంతమైన ఇంప్లాంటేషన్ మరియు గర్భధారణకు ఉత్తమ అవకాశం కోసం, FETకు ముందు మరియు తర్వాత మద్యపానానికి దూరంగా ఉండటం ముఖ్యం.
Whether a hormonal imbalance can be “cured” depends on the cause. If its due to lifestyle factors like diet or stress, making lasting changes can restore balance permanently. However, for medical conditions like PCOS or thyroid disorders, its about lifelong management with medication and lifestyle, not a one-time cure.
Hormone levels are checked through a simple blood test ordered by your doctor. The doctor will test for specific hormones like thyroid (TSH, T3, T4), estrogen, progesterone, and testosterone based on your symptoms.
A balanced diet is key. Focus on:
- Healthy Fats: Nuts, seeds (like flax and chia), and avocado.
- Lean Protein: Lentils (dal), chickpeas, eggs, and fish.
- High-Fiber Foods: Leafy green vegetables (palak), broccoli, and whole grains.
Antioxidant-Rich Foods: Berries, pomegranates, and colourful vegetables.
No single fruit is a magic cure. The best approach is to eat a variety of fruits. Berries (like strawberries and blueberries) are excellent for their antioxidants, and avocados are great for their healthy fats which are building blocks for hormones.
A combination of different exercises is most effective:
- Yoga and Walking: To reduce stress and lower cortisol levels.
- Strength Training: To build muscle and improve insulin sensitivity.
- Moderate Cardio: To maintain a healthy weight.
Yes, it is possible, but a hormonal imbalance can make it more difficult. Imbalances can interfere with ovulation (the release of an egg), making conception challenging. Treating the underlying hormonal issue is often the first and most important step to improving fertility.
You can naturally support your hormonal health by focusing on four key areas:
- Diet: Eat whole, unprocessed foods.
- Exercise: Get regular, moderate physical activity.
- Stress: Manage stress through meditation, deep breathing, or hobbies.
- Sleep: Prioritize getting 7-8 hours of quality sleep per night.
No. The only difference is that conception happens in a lab instead of the fallopian tube. Once the embryo is in the womb, the pregnancy and the child’s development are the same.
No, this is a common myth. Studies have proven that IVF babies are just as strong and healthy as babies conceived naturally.
IVF children do not have any unique health problems. Their risk of common childhood illnesses is the same as that of the general population.
Absolutely not. The method of conception has no impact on a child’s intelligence.
No. They inherit their physical traits from their parents genes, just like any other child.
This used to be more common, but with modern technology and the preference for single embryo transfer at centres like Ferty9, the chance of having multiples is now much lower and closer to that of natural conception.
Cramping after IUI can happen due to the procedure itself or ovulation. It’s not always a sure sign of pregnancy.
Cramping may occur because of the catheter insertion, mild uterine irritation, or natural ovulation-related changes.
అవును, IUI తర్వాత లైంగిక కార్యకలాపాలు గర్భధారణ అవకాశాలను మెరుగుపరుస్తాయని పరిశోధనలు సూచిస్తున్నాయి. వీర్యంలోని సహజ ప్రోస్టాగ్లాండిన్లు ఈ క్రింది విధంగా సహాయపడతాయి:
- కటి ప్రాంతానికి రక్త ప్రసరణను మెరుగుపరుస్తాయి.
- ప్రయోజనకరమైన గర్భాశయ సంకోచాలను సృష్టిస్తాయి.
- అండం వైపు శుక్రకణాల కదలికకు మద్దతు ఇస్తాయి.
- గర్భాశయ ముఖద్వారం మృదువుగా మారడానికి సహాయపడతాయి.
ఇది తప్పనిసరి కాదు, కానీ IUI తర్వాత లైంగిక సంబంధాలు ప్రక్రియకు సహాయపడగలవు. జంటలు సాధారణ లైంగిక సంబంధాలను కొనసాగించమని డాక్టర్లు తరచుగా ప్రోత్సహిస్తారు, ఎందుకంటే ఇది:
- ఒత్తిడి స్థాయిలను తగ్గిస్తుంది.
- భాగస్వాముల మధ్య భావోద్వేగ బంధాన్ని కొనసాగిస్తుంది.
- ఫలదీకరణ కోసం అదనపు శుక్రకణాలను అందిస్తుంది.
- మొత్తం చికిత్సా విజయానికి మద్దతు ఇస్తుంది.
వైద్య మార్గదర్శకాలను అనుసరించినప్పుడు, IUI తర్వాత లైంగిక సంబంధాలు సాధారణంగా సురక్షితమైనవి. అయితే, లైంగిక కార్యకలాపాలను పునఃప్రారంభించే ముందు రోగులు ప్రక్రియ తర్వాత కనీసం 18–24 గంటలు వేచి ఉండాలి. ఈ నిరీక్షణ కాలం గర్భాశయ ముఖద్వారం సరిగ్గా మూసుకోవడానికి మరియు ఇన్ఫెక్షన్ ప్రమాదాన్ని తగ్గించడానికి సహాయపడుతుంది. ఈ కాలంలో తేలికపాటి స్పాటింగ్ సాధారణం, కానీ రోగులు గణనీయమైన అసౌకర్యం లేదా అసాధారణ లక్షణాలను అనుభవిస్తే వారి ఫెర్టిలిటీ నిపుణులను సంప్రదించాలి.
కొంతమంది రోగులకు, ఉష్ణోగ్రతను ట్రాక్ చేయడం వారి చికిత్సలో నియంత్రణ మరియు భాగస్వామ్యం అనే భావనను అందిస్తుంది. అయితే, మరికొందరిలో ఇది అనవసరమైన ఆందోళనను పెంచవచ్చు. BBT పర్యవేక్షణ ప్రయోజనకరంగా ఉంటుందో లేదో తెలుసుకోవడానికి మీ ఆరోగ్య బృందంతో చర్చించాలని డాక్టర్లు సిఫార్సు చేస్తారు.
IVF సమయంలో BBT ట్రాకింగ్ తప్పనిసరి కాదు. కొంతమంది రోగులు చికిత్సకు తమ శరీరం యొక్క స్పందనను అర్థం చేసుకోవడానికి ఇది సహాయకరంగా ఉంటుందని భావిస్తారు. కానీ, చికిత్సా నిర్ణయాల కోసం డాక్టర్లు ప్రాథమికంగా రక్త పరీక్షలు మరియు అల్ట్రాసౌండ్స్ వంటి ఇతర పర్యవేక్షణ పద్ధతులపై దృష్టి పెడతారు.
అవును, IVF మందులు ఉష్ణోగ్రత సరళిని గణనీయంగా ప్రభావితం చేస్తాయి:
- స్టిమ్యులేషన్ మందులు: ప్రాథమిక ఉష్ణోగ్రతను పెంచగలవు.
- ప్రొజెస్టెరాన్ సప్లిమెంట్స్: పెరిగిన ఉష్ణోగ్రత అలాగే కొనసాగేలా చేస్తాయి.
- ట్రిగ్గర్ షాట్స్: తాత్కాలికంగా ఉష్ణోగ్రతను పెంచుతాయి.
- సహాయక మందులు: సహజమైన ఉష్ణోగ్రత సరళిని కప్పిపుచ్చవచ్చు.
పిండ బదిలీ తర్వాత ఉష్ణోగ్రతను ట్రాక్ చేయడం సిఫార్సు చేయబడదు, ఎందుకంటే ఇది అనవసరమైన ఒత్తిడికి కారణం కావచ్చు. ఈ దశలో ఉపయోగించే మందులు ఉష్ణోగ్రత సరళిని ప్రభావితం చేస్తాయి, అందువల్ల ఈ రీడింగ్లు చికిత్స విజయాన్ని సూచించే నమ్మదగని సూచికలు.
IVF చికిత్సలో ఉపయోగించే మందులు సహజమైన ఉష్ణోగ్రత సరళిని మారుస్తాయి కాబట్టి, BBT పర్యవేక్షణ ఓవులేషన్ను కచ్చితంగా అంచనా వేయలేదు. అండాల పెరుగుదలను గమనించడానికి మరియు అండాల సేకరణకు సరైన సమయాన్ని నిర్ధారించడానికి డాక్టర్లు రక్త పరీక్షలు మరియు అల్ట్రాసౌండ్ పర్యవేక్షణపై ఆధారపడతారు.
Laser assisted hatching is a technique used in IVF treatment that creates an opening in the zona pellucida (outer shell) of the embryo. The laser beam pulses three times to create a complete gap, allowing embryonic cells to hatch and improve implantation into the uterine lining without affecting the embryo.
Patients over 37 years old, those with frozen embryo replacement (FER), couples who experienced failed IVF/ICSI cycles, poor responders requiring high doses of gonadotropins, those with poor quality embryos, low fertilization rates in previous cycles, and embryos with thick zona pellucida.
Laser assisted hatching is safer than chemical and manual hatching methods, requires fewer embryos to be transferred, increases implantation success rates, reduces chances of multiple pregnancies, and provides better control over opening size compared to mechanical methods using micro-needles.
Unlike chemical hatching which uses Tyrode’s acid and risks chemical exposure to embryos, laser assisted hatching uses an invisible laser beam to dissolve the shell precisely. It eliminates chemical exposure risks and provides exact technique control based on power settings and spot size.
Success depends significantly on the embryologist’s experience and technique execution. The procedure has improved clinical pregnancy rates when performed correctly. The laser technique may vary based on power settings, spot size, and the specific approach used by the experienced embryologist.
Ideal IVF candidates include women with blocked fallopian tubes, endometriosis, PCOS, irregular ovulation cycles, male factor infertility cases, and couples with unexplained infertility. Women with fertility disorders benefit significantly as fertility drugs can induce ovulation and generate healthy eggs.
Women over 37 may experience decreased IVF effectiveness, though age alone doesn’t disqualify all candidates. Those unable to produce healthy eggs may need donor options. Women not interested in using donor eggs when their own aren’t viable may not benefit from IVF.
IVF involves retrieving and fertilizing sperm and egg outside the body, developing them into embryos, then transferring embryos into the woman’s uterus for implantation. The process can use the couple’s own gametes or donor eggs/sperm depending on individual needs.
IVF allows sperm to naturally fertilize eggs in laboratory conditions, while ICSI involves directly injecting a single sperm into an egg. ICSI can improve success chances when male factor infertility is the main challenge, offering better fertilization control.
Yes, couples with unexplained infertility are good IVF candidates. These couples have undergone testing without finding specific fertility issues but struggle with natural conception, fertility medications, or IUI. IVF offers them a viable path to parenthood.
In-vitro maturation (IVM) is a breakthrough fertility treatment using 90% fewer hormones than IVF. Instead of stimulating eggs with heavy hormone doses, IVM retrieves immature eggs and matures them in the laboratory using special proteins like cumin and cAMP modulators.
IVM offers reduced hormone exposure (90% less), costs one-third to half of IVF expenses, fewer medical complications, safer treatment for PCOS patients, less monitoring (1-2 ultrasounds vs 3+ for IVF), and eliminates ovarian hyperstimulation syndrome risks completely.
IVM eliminates the risk of ovarian hyperstimulation syndrome, a serious complication particularly affecting PCOS patients. The significantly reduced hormone requirement makes it ideal for the 10-15% of women with polycystic ovarian syndrome who face higher risks with traditional IVF.
Current IVM limitations include lower efficiency in producing good-quality embryos compared to IVF, and many clinicians preferring IVF for maximizing pregnancy chances. However, improved IVM techniques show 50% increase in embryo numbers and better quality than standard IVM.
The improved IVM treatment is moving into preclinical trial phases. Once approved by regulatory bodies, this advanced fertility treatment could become available to women within 3-5 years, potentially becoming a common alternative to traditional IVF.
Yes, normal delivery is absolutely possible after IVF pregnancy. The mode of delivery depends on factors like mother’s health, baby’s position, pregnancy progress, and underlying conditions. Many IVF women successfully deliver vaginally despite common misconceptions requiring cesarean sections.
Key factors include maternal age, single vs multiple pregnancies, baby’s position, placental health, underlying conditions like hypertension or diabetes, fetal health concerns, and previous C-section history. Advanced maternal age and multiples may increase C-section likelihood.
Benefits include quicker recovery compared to C-section, lower surgical risks and infection rates, emotional satisfaction, immediate mother-baby bonding, enhanced early breastfeeding opportunities, and shorter hospital stays allowing faster return home with the newborn.
Risks include higher chance of preterm labor, increased placental issues like placenta previa or abruption, and potential complications from previous uterine surgeries. However, with advanced medical care and constant monitoring, these risks can be effectively managed.
C-section becomes essential for multiple pregnancies (twins/triplets), breech or transverse baby positions, health complications like preeclampsia or gestational diabetes, and labor complications including prolonged or stalled labor. Safety of mother and baby is always the priority.
Yes, couples can successfully conceive after multiple failures. Mrs. Riya (39) and Mr. Santosh’s case demonstrates that even after 4 failed cycles with low AMH (0.5 ng/mL), pregnancy is possible through optimized treatment protocols and advanced techniques at specialized centers.
Advanced techniques include pre-treatment to improve ovarian response, embryo pooling to increase success chances, Preimplantation Genetic Testing for Aneuploidy (PGTA) for selecting normal embryos, hysteroscopic polypectomy to remove polyps, and natural cycle endometrial preparation for optimal implantation.
Low AMH (0.5 ng/mL) indicates reduced ovarian reserve, making conception challenging especially at age 39. However, with modified IVF programs including pre-treatment protocols and embryo pooling techniques, successful pregnancy is still achievable through personalized medical approaches.
Embryo pooling involves collecting multiple embryos across several cycles to increase viable embryo numbers. This technique particularly benefits women with low ovarian reserve, maximizing chances of finding genetically normal embryos for successful transfer and implantation.
While donor eggs remain an option, couples can still attempt conception with their own gametes using advanced techniques. Mrs. Riya and Mr. Santosh were determined to use their own gametes and succeeded, proving donor eggs aren’t always necessary.
IVF involves ovarian stimulation, egg retrieval, fertilization in laboratory, embryo culture, and transfer. The process typically takes 4-6 weeks with careful monitoring at each stage to optimize success rates and ensure patient safety throughout treatment.
Preparation includes comprehensive fertility testing, lifestyle optimization, nutritional supplementation, medication protocols, and psychological counseling. Pre-treatment evaluation helps identify and address factors that could affect treatment success.
Understanding the four stages of embryonic development is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of the four stages of embryonic development on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for the four stages of embryonic development range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding frozen embryo transfer advantages and considerations is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of frozen embryo transfer advantages and considerations on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for frozen embryo transfer advantages and considerations range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding blastocyst culture stages and significance is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of blastocyst culture stages and significance on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Understanding facts and tips for fertility treatments is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of facts and tips for fertility treatments on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for facts and tips for fertility treatments range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding tips for pregnancy after fertility treatments is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of tips for pregnancy after fertility treatments on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for tips for pregnancy after fertility treatments range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding public view and assumptions towards fertility treatments is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of public view and assumptions towards fertility treatments on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for public view and assumptions towards fertility treatments range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding ferty9 and its blastocyst culture is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of ferty9 and its blastocyst culture on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for ferty9 and its blastocyst culture range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding lets celebrate embryologist day 2024 with ferty9 is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of lets celebrate embryologist day 2024 with ferty9 on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Treatment approaches for lets celebrate embryologist day 2024 with ferty9 range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Main causes include ovulation disorders, blocked fallopian tubes, endometriosis, uterine abnormalities, age-related factors, and hormonal imbalances. Each condition requires specific diagnostic approaches and treatment strategies.
Assessment includes hormone testing, ovarian reserve evaluation, pelvic ultrasound, hysterosalpingogram, and sometimes laparoscopy. Comprehensive testing identifies specific factors affecting fertility.
Treatments include ovulation induction, surgical procedures, lifestyle modifications, and assisted reproductive technologies. Treatment choice depends on diagnosis, age, and individual circumstances.
Age significantly affects egg quality and quantity, with fertility declining after age 35 and more rapidly after 40. Earlier intervention and fertility preservation options can help address age-related challenges.
Natural optimization includes maintaining healthy weight, balanced nutrition, regular exercise, stress management, avoiding harmful substances, and tracking ovulation cycles for optimal timing.
Understanding music therapy for fertility with ferty9 is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of music therapy for fertility with ferty9 on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for music therapy for fertility with ferty9 range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding genetic testing costs in fertility treatment pgspgt is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of genetic testing costs in fertility treatment pgspgt on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for genetic testing costs in fertility treatment pgspgt range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding anorexia bulimia how eating disorders affect fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
The impact of anorexia bulimia how eating disorders affect fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Treatment approaches for anorexia bulimia how eating disorders affect fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what is ferty 9 approach all about is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what is ferty 9 approach all about on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what is ferty 9 approach all about range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding can stis impact fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of can stis impact fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for can stis impact fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding antioxidants and fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of antioxidants and fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for antioxidants and fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding the role of probiotics in fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of the role of probiotics in fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for the role of probiotics in fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding a fertility clinic and fertility specialists is crucial for making informed decisions about fertility. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The fertility treatment varies by individual factors, including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Common causes include varicocele, hormonal imbalances, genetic factors, infections, lifestyle factors, and environmental exposures. Approximately 40-50% of infertility cases involve male factors, making comprehensive evaluation essential for couples.
Evaluation includes semen analysis, hormone testing, physical examination, genetic testing, and imaging studies when indicated. Detailed assessment helps identify specific causes and guide appropriate treatment strategies.
Treatments range from lifestyle modifications and medications to surgical interventions and assisted reproductive techniques. Options include varicocele repair, hormone therapy, sperm retrieval procedures, and ICSI for severe cases.
Lifestyle factors significantly impact sperm quality including diet, exercise, smoking, alcohol consumption, stress levels, and environmental exposures. Optimizing these factors can improve fertility outcomes naturally.
Men should seek evaluation after 6-12 months of unsuccessful conception attempts, or immediately if known risk factors exist like previous surgeries, infections, or medical conditions affecting fertility.
Understanding how is primary care bottom line option for boosting fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how is primary care bottom line option for boosting fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how is primary care bottom line option for boosting fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 17 top organic foods which support fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 17 top organic foods which support fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 17 top organic foods which support fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding triphasic bbt chart causes triphasic temperature shifts is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of triphasic bbt chart causes triphasic temperature shifts on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for triphasic bbt chart causes triphasic temperature shifts range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding misconceptions about fertility what to know is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of misconceptions about fertility what to know on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for misconceptions about fertility what to know range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding managing chronic health for fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of managing chronic health for fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for managing chronic health for fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding surprising facts of fertility treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of surprising facts of fertility treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for surprising facts of fertility treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding understanding the different types of fertility medications is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of understanding the different types of fertility medications on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for understanding the different types of fertility medications range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 8 surprising facts about fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 8 surprising facts about fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 8 surprising facts about fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding why ferty9 is the best fertility clinic in kukatpally is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of why ferty9 is the best fertility clinic in kukatpally on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for why ferty9 is the best fertility clinic in kukatpally range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 5 techniques to improve fertility treatment in hyderabad is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 5 techniques to improve fertility treatment in hyderabad on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 5 myths about fertility one needs to stop at once is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 5 myths about fertility one needs to stop at once on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 5 myths about fertility one needs to stop at once range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding balancing act tips for maintaining fertility while pursuing a fitness passion is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of balancing act tips for maintaining fertility while pursuing a fitness passion on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for balancing act tips for maintaining fertility while pursuing a fitness passion range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding role of fertility centers in modern reproductive medicine is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of role of fertility centers in modern reproductive medicine on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for role of fertility centers in modern reproductive medicine range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding when is a fertility check required is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of when is a fertility check required on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for when is a fertility check required range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding impact of body mass index on fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of impact of body mass index on fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for impact of body mass index on fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding advances in fertility treatment allow people to have children with limits is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of advances in fertility treatment allow people to have children with limits on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for advances in fertility treatment allow people to have children with limits range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 7 things to consider when choosing a fertility clinic is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 7 things to consider when choosing a fertility clinic on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 7 things to consider when choosing a fertility clinic range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 10 ways to stay fertile your 30s is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 10 ways to stay fertile your 30s on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 10 ways to stay fertile your 30s range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding overcoming fertility challenges with age is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of overcoming fertility challenges with age on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for overcoming fertility challenges with age range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding unknown zika facts make it scarier is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of unknown zika facts make it scarier on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for unknown zika facts make it scarier range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding understanding the importance of fertility awareness is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of understanding the importance of fertility awareness on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for understanding the importance of fertility awareness range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding regenerative medicine and its therapies is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of regenerative medicine and its therapies on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for regenerative medicine and its therapies range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding when check up required with vijayawada fertility doctor is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of when check up required with vijayawada fertility doctor on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for when check up required with vijayawada fertility doctor range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding primary care the bottom line option for boosting fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of primary care the bottom line option for boosting fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for primary care the bottom line option for boosting fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding new stem cell research aims to stop boys losing fertility after childhood cancer treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of new stem cell research aims to stop boys losing fertility after childhood cancer treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for new stem cell research aims to stop boys losing fertility after childhood cancer treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how many eggs does a woman have is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how many eggs does a woman have on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how many eggs does a woman have range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how pcod does affect fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how pcod does affect fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how pcod does affect fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding doctors are good teachers when patients ask good questions is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of doctors are good teachers when patients ask good questions on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for doctors are good teachers when patients ask good questions range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding does age affect fertility treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of does age affect fertility treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for does age affect fertility treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding double marker test procedure is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of double marker test procedure on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for double marker test procedure range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding the role of genetics in recurrent implantation failures is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of the role of genetics in recurrent implantation failures on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for the role of genetics in recurrent implantation failures range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding overcoming fertility challenges with donor eggs is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of overcoming fertility challenges with donor eggs on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for overcoming fertility challenges with donor eggs range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding key differences between cryopreservation and freezing is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of key differences between cryopreservation and freezing on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for key differences between cryopreservation and freezing range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding compare and choose best imsi ivm treatment in hyderabad is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of compare and choose best imsi ivm treatment in hyderabad on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for compare and choose best imsi ivm treatment in hyderabad range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding increase progesterone naturally tips is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of increase progesterone naturally tips on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for increase progesterone naturally tips range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding cervical mucus to predict ovulation is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of cervical mucus to predict ovulation on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for cervical mucus to predict ovulation range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding impact of stress on fertility strategies for stress reduction during treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of impact of stress on fertility strategies for stress reduction during treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for impact of stress on fertility strategies for stress reduction during treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding is stress really affecting ones fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of is stress really affecting ones fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for is stress really affecting ones fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding impact of stress and its effects on sexual health is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of impact of stress and its effects on sexual health on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for impact of stress and its effects on sexual health range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding tips for maintaining optimal vaginal hygiene is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of tips for maintaining optimal vaginal hygiene on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for tips for maintaining optimal vaginal hygiene range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how to reduce sgpt and sgot levels is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how to reduce sgpt and sgot levels on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how to reduce sgpt and sgot levels range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding pelvic exercise effective in premature ejaculation is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of pelvic exercise effective in premature ejaculation on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for pelvic exercise effective in premature ejaculation range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding treatment for teratozoospermia is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of treatment for teratozoospermia on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for treatment for teratozoospermia range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding teratozoospermia causes and treatment options to consider is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of teratozoospermia causes and treatment options to consider on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for teratozoospermia causes and treatment options to consider range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding know more about pcod problem and pcod remedies is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of know more about pcod problem and pcod remedies on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for know more about pcod problem and pcod remedies range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 10 ways how to avoid cesarean section is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 10 ways how to avoid cesarean section on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 10 ways how to avoid cesarean section range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what to do when failed copper t results in unwanted pregnancy is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what to do when failed copper t results in unwanted pregnancy on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what to do when failed copper t results in unwanted pregnancy range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding steps to prepare for pregnancy following a miscarriage is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of steps to prepare for pregnancy following a miscarriage on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for steps to prepare for pregnancy following a miscarriage range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding the path from zygote to baby is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of the path from zygote to baby on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for the path from zygote to baby range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding vaginal swelling during pregnancy is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of vaginal swelling during pregnancy on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for vaginal swelling during pregnancy range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding endometrium thickness in pregnancy symptoms treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of endometrium thickness in pregnancy symptoms treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for endometrium thickness in pregnancy symptoms treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding get pregnant after early menopause is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of get pregnant after early menopause on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for get pregnant after early menopause range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding irregular periods pregnancy tips is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of irregular periods pregnancy tips on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for irregular periods pregnancy tips range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding can progesterone improve chances of pregnancy is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of can progesterone improve chances of pregnancy on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for can progesterone improve chances of pregnancy range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding can losing weight increase your chances of getting pregnant is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of can losing weight increase your chances of getting pregnant on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for can losing weight increase your chances of getting pregnant range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding be positive mothers to be is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of be positive mothers to be on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for be positive mothers to be range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 3 common pregnancy accidents and how handle them is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 3 common pregnancy accidents and how handle them on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 3 common pregnancy accidents and how handle them range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding pregnancy complications to know about is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of pregnancy complications to know about on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for pregnancy complications to know about range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding normal delivery vs cesarean is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of normal delivery vs cesarean on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for normal delivery vs cesarean range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
The impact of what if a missed period is still a negative pregnancy test 2 on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding post natal care dos and donts after normal delivery is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of post natal care dos and donts after normal delivery on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for post natal care dos and donts after normal delivery range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 16 useful trying to conceive tips from experts is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 16 useful trying to conceive tips from experts on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 16 useful trying to conceive tips from experts range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding understanding recurrent pregnancy loss is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of understanding recurrent pregnancy loss on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for understanding recurrent pregnancy loss range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding late age pregnancy risks and challenges is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of late age pregnancy risks and challenges on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for late age pregnancy risks and challenges range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding pros and cons of getting pregnant your 20s 30s and 40s is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of pros and cons of getting pregnant your 20s 30s and 40s on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for pros and cons of getting pregnant your 20s 30s and 40s range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding laparoscopy can be the need of the hour is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of laparoscopy can be the need of the hour on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for laparoscopy can be the need of the hour range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding side effects of preponing vs postponing menstrual pill is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of side effects of preponing vs postponing menstrual pill on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for side effects of preponing vs postponing menstrual pill range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding symptoms of blocked fallopian tubes is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of symptoms of blocked fallopian tubes on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for symptoms of blocked fallopian tubes range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding signs and symptoms after uterine evaluation treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of signs and symptoms after uterine evaluation treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for signs and symptoms after uterine evaluation treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 8 important tips to choose the best gynecologist is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 8 important tips to choose the best gynecologist on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 8 important tips to choose the best gynecologist range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Understanding benefits side effects of contraceptive sponge is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of benefits side effects of contraceptive sponge on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for benefits side effects of contraceptive sponge range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding irregular periods a warning sign to be taken care of is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of irregular periods a warning sign to be taken care of on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for irregular periods a warning sign to be taken care of range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Immediately after your transfer, you should avoid processed junk foods, high-mercury or raw fish, unpasteurized dairy products (like raw milk or cheese), undercooked meats, all alcoholic beverages, and foods high in refined sugar (like sweets and sodas).
It is important to avoid high-mercury fish because mercury is a heavy metal that can be harmful to a developing baby’s brain and nervous system, especially in the very early stages of pregnancy.
Yes, but in moderation. It is recommended to limit your caffeine intake to less than 200 mg per day, which is about one small cup of coffee or two cups of tea. Excessive caffeine may interfere with the embryos ability to implant successfully.
Not all herbal teas and supplements are safe. Some herbs can interfere with your hormones or even cause uterine contractions, which you want to avoid. It is crucial to consult your doctor at Ferty9 before taking any herbal products or supplements.
Focus on a balanced, nourishing diet. Excellent choices include:
- Lean Proteins: Lentils (dal), chickpeas, eggs, and well-cooked chicken.
- Healthy Fats: Walnuts, chia seeds, flaxseeds, and avocado.
- Leafy Greens: Spinach (palak) and other local greens.
- Whole Grains: Brown rice, oats, and whole wheat roti.
- Fruits: Berries, pomegranates, and bananas.
Understanding iui procedure preparation success factors is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of iui procedure preparation success factors on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for iui procedure preparation success factors range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how many eggs does a woman have telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how many eggs does a woman have telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how many eggs does a woman have telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding genital tuberculosis symptoms treatment fertility impact telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of genital tuberculosis symptoms treatment fertility impact telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for genital tuberculosis symptoms treatment fertility impact telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding genital tuberculosis symptoms treatment fertility impact is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of genital tuberculosis symptoms treatment fertility impact on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for genital tuberculosis symptoms treatment fertility impact range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 10 tips to get prepared for the babys arrival is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 10 tips to get prepared for the babys arrival on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 10 tips to get prepared for the babys arrival range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding side effects of preponing vs postponing menstrual pill in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of side effects of preponing vs postponing menstrual pill in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for side effects of preponing vs postponing menstrual pill in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding treatment for teratozoospermia in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of treatment for teratozoospermia in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for treatment for teratozoospermia in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding iui and beginners guide to intrauterine insemination is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of iui and beginners guide to intrauterine insemination on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for iui and beginners guide to intrauterine insemination range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding iui procedure telugu preparation success factors is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of iui procedure telugu preparation success factors on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for iui procedure telugu preparation success factors range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding endometrium thickness in pregnancy symptoms treatment in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of endometrium thickness in pregnancy symptoms treatment in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for endometrium thickness in pregnancy symptoms treatment in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
The provided context does not contain information on the specific quantity of sperm needed for pregnancy. It emphasizes the importance of sperm quality, including movement (motility) and shape (morphology).
According to the information, it is a myth that sperm can reliably survive for 7 days. While extremely rare instances might be theoretically possible, sperm typically live for 2 to 3 days, and up to a maximum of 5 days under ideal fertile conditions.
Sperm can wait for an egg for the duration of their lifespan within the female body. In a fertile environment, this can be up to 5 days. This is why having intercourse in the few days leading up to ovulation can lead to pregnancy, as the sperm can wait for the egg to be released.
Understanding comparing choosing best icsi imsi treatment in hyderabad is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of comparing choosing best icsi imsi treatment in hyderabad on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for comparing choosing best icsi imsi treatment in hyderabad range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding prepare for first fertility preservation consultation is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of prepare for first fertility preservation consultation on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for prepare for first fertility preservation consultation range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding tips and support for fertility preservation decisions is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of tips and support for fertility preservation decisions on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for tips and support for fertility preservation decisions range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding fertility preservation options and importance is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of fertility preservation options and importance on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for fertility preservation options and importance range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding importance of fertility preservation in family planning is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of importance of fertility preservation in family planning on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for importance of fertility preservation in family planning range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding helping couple overcome multiple infertility challenges is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of helping couple overcome multiple infertility challenges on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for helping couple overcome multiple infertility challenges range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding causes and treatment options for unexplained infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of causes and treatment options for unexplained infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for causes and treatment options for unexplained infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 5 ways of coping up with infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 5 ways of coping up with infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 5 ways of coping up with infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Understanding reasons to consider an infertility evaluation is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of reasons to consider an infertility evaluation on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for reasons to consider an infertility evaluation range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding reducing the risk of infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of reducing the risk of infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for reducing the risk of infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding basic check list for disposing infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of basic check list for disposing infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for basic check list for disposing infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 7 tips that can help find the best infertility centre is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 7 tips that can help find the best infertility centre on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 7 tips that can help find the best infertility centre range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding best ways to overcome infertility due thyroid is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of best ways to overcome infertility due thyroid on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Treatment approaches for best ways to overcome infertility due thyroid range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 10 myths common with infertility treatment in hyderabad is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 10 myths common with infertility treatment in hyderabad on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Treatment approaches for 10 myths common with infertility treatment in hyderabad range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how to get pregnant lifestyle tips for fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how to get pregnant lifestyle tips for fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how to get pregnant lifestyle tips for fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how to face mothers day when coping with infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how to face mothers day when coping with infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how to face mothers day when coping with infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how to cope the emotional crisis of infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how to cope the emotional crisis of infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how to cope the emotional crisis of infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding uterine and cervical problems that lead to infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of uterine and cervical problems that lead to infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for uterine and cervical problems that lead to infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 1 in 6 people globally affected by infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 1 in 6 people globally affected by infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 1 in 6 people globally affected by infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what if a missed period is still a negative pregnancy test in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what if a missed period is still a negative pregnancy test in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what if a missed period is still a negative pregnancy test in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding the path from zygote to baby in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of the path from zygote to baby in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for the path from zygote to baby in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what is implantation bleeding is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what is implantation bleeding on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what is implantation bleeding range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what to do cum after embryo transfer is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what to do cum after embryo transfer on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what to do cum after embryo transfer range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding benefits and limitations of iui treatment is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of benefits and limitations of iui treatment on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for benefits and limitations of iui treatment range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how does the best fertility center in hyderabad look like is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how does the best fertility center in hyderabad look like on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how does the best fertility center in hyderabad look like range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding choosing hospital infertility treatment in hyderabad is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of choosing hospital infertility treatment in hyderabad on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for choosing hospital infertility treatment in hyderabad range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding role of regular health screenings in maintaining fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of role of regular health screenings in maintaining fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for role of regular health screenings in maintaining fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding ones customers view about the best hospital in kukatpally is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of ones customers view about the best hospital in kukatpally on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for ones customers view about the best hospital in kukatpally range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what do cramps after iui indicate in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what do cramps after iui indicate in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what do cramps after iui indicate in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how soon can a pregnancy test be taken after implantation in telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how soon can a pregnancy test be taken after implantation in telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how soon can a pregnancy test be taken after implantation in telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding andropause causes symptoms treatment diagnosis is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of andropause causes symptoms treatment diagnosis on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for andropause causes symptoms treatment diagnosis range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 5 tips for how to increase ovulation naturally is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 5 tips for how to increase ovulation naturally on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 5 tips for how to increase ovulation naturally range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding fertility preservation options for cancer is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of fertility preservation options for cancer on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for fertility preservation options for cancer range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding do overeating obesity stress anxiety impact fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of do overeating obesity stress anxiety impact fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for do overeating obesity stress anxiety impact fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
ప్రతి వ్యక్తి సంతానోత్పత్తి ప్రయాణం ప్రత్యేకమైనదని మేము అర్థం చేసుకున్నాము. సమగ్ర మూల్యాంకనం తర్వాత, మా నిపుణుల బృందం ప్రతి పురుషుని ప్రత్యేక అవసరాలు మరియు పరిస్థితులకు తగినట్లుగా వ్యక్తిగత చికిత్సా ప్రణాళికను అభివృద్ధి చేస్తుంది.
ఆండ్రోమాక్స్ వీర్య విశ్లేషణ, హార్మోన్ల పరీక్ష, మరియు జన్యుపరమైన స్క్రీనింగ్ వంటి అధునాతన రోగ నిర్ధారణ పద్ధతులను ఉపయోగిస్తుంది. అదనంగా, అవసరమైనప్పుడు ఇది IVF లేదా IUI వంటి ఇతర సంతానోత్పత్తి టెక్నాలజీలతో సజావుగా కలిసిపోతుంది, గర్భధారణ అవకాశాలను పెంచుతుంది.
ఆండ్రోమాక్స్ తక్కువ శుక్రకణాల సంఖ్య, తక్కువ శుక్రకణాల కదలిక, అసాధారణ శుక్రకణాల ఆకృతి, హార్మోన్ల అసమతుల్యతలు, జన్యుపరమైన కారకాలు, మరియు జీవనశైలికి సంబంధించిన సంతానలేమి కారకాలతో సహా విస్తృత శ్రేణి పురుషుల సంతానోత్పత్తి సమస్యలను పరిష్కరిస్తుంది.
ఆండ్రోమాక్స్ పురుషుల సంతానోత్పత్తికి ఒక సమగ్ర మరియు వ్యక్తిగత విధానాన్ని అందించడం ద్వారా ప్రత్యేకంగా నిలుస్తుంది. ఇది అధునాతన రోగ నిర్ధారణ, అనుకూలీకరించిన చికిత్సా ప్రణాళికలు, పోషకాహార మద్దతు, మరియు జీవనశైలి కోచింగ్ను మిళితం చేస్తుంది.
Understanding 5 signs of implantation before a positive pregnancy test is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 5 signs of implantation before a positive pregnancy test on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 5 signs of implantation before a positive pregnancy test range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding blood test after implantation pregnancy detection is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of blood test after implantation pregnancy detection on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for blood test after implantation pregnancy detection range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding pregnancy test after implantation is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of pregnancy test after implantation on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for pregnancy test after implantation range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding implantation bleeding vs period differences is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of implantation bleeding vs period differences on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for implantation bleeding vs period differences range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding top factors affecting iui success rate is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of top factors affecting iui success rate on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for top factors affecting iui success rate range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Sperm morphology refers to the size and shape of sperm cells. According to WHO 2025 criteria, normal sperm morphology ranges from 4-14%. Even men with only 4% normal-shaped sperm can achieve successful fertilization.
According to WHO 2025 criteria, 4-14% normal forms indicate good fertility potential.
Yes, through lifestyle changes, IUI, IVF, and ICSI with success rates of 15-70%.
Genetic factors, infections, varicocele, smoking, alcohol, stress, and toxin exposure.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Yes, but you should drink it in moderation. Its best to limit your total caffeine intake to less than 200 mg per day. This is roughly equal to one small cup of coffee or two cups of tea. Too much caffeine may affect blood flow to the uterus, so its safer to keep your intake low.
Focus on a variety of fresh, seasonal fruits. Berries (like strawberries and blueberries), pomegranates, and bananas are excellent choices because they are rich in antioxidants and vitamins. A colorful fruit bowl will provide a wide range of nutrients to support your body.
Yes, you should continue taking any prenatal vitamins that your Ferty9 doctor has prescribed, especially those containing folic acid. However, do not start any new supplements or herbal remedies without first consulting your doctor.
You should follow this supportive, healthy diet throughout the entire two-week wait. If your pregnancy test is positive, its a great idea to continue these healthy eating habits into your pregnancy to support both your health and your babys development.
It’s best to be cautious with spicy food. While mild, home-cooked spicy food is generally fine, it’s wise to avoid very hot or oily dishes. These can sometimes cause indigestion or stomach discomfort, which you want to avoid during this sensitive time. Stick to meals that are warm, comforting, and easy to digest.
Understanding what are follicles and why are they important for fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what are follicles and why are they important for fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what are follicles and why are they important for fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding antidepressants and its impact on fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of antidepressants and its impact on fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for antidepressants and its impact on fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding can music improve embryo implantation is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of can music improve embryo implantation on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for can music improve embryo implantation range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how long after implantation bleeding can i test is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how long after implantation bleeding can i test on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how long after implantation bleeding can i test range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding how to choose the best fertility treatment method is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of how to choose the best fertility treatment method on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for how to choose the best fertility treatment method range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding connection between gut health and fertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of connection between gut health and fertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for connection between gut health and fertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what to expect in infertility counselling is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what to expect in infertility counselling on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what to expect in infertility counselling range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding can stress cause infertility is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of can stress cause infertility on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for can stress cause infertility range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding follicular monitoring procedure how it works is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of follicular monitoring procedure how it works on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for follicular monitoring procedure how it works range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding teratozoo spermia lifestyle changes and fertility tips is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of teratozoo spermia lifestyle changes and fertility tips on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for teratozoo spermia lifestyle changes and fertility tips range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding is picsi right for you is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of is picsi right for you on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for is picsi right for you range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding ferty9 helps couple conceive by overcoming genetic condition is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of ferty9 helps couple conceive by overcoming genetic condition on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for ferty9 helps couple conceive by overcoming genetic condition range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding teratozoospermia causes symptoms diagnosis is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of teratozoospermia causes symptoms diagnosis on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for teratozoospermia causes symptoms diagnosis range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what are follicles and why are they important for fertility telugu is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what are follicles and why are they important for fertility telugu on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what are follicles and why are they important for fertility telugu range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding what if a missed period is still a negative pregnancy test is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of what if a missed period is still a negative pregnancy test on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for what if a missed period is still a negative pregnancy test range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding 15 little tips to care for your uterus is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of 15 little tips to care for your uterus on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for 15 little tips to care for your uterus range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
Understanding pregnancy test one day after implantation bleeding is important for making informed fertility decisions. This condition/procedure requires comprehensive evaluation by fertility specialists to determine appropriate treatment approaches and expected outcomes based on individual circumstances.
The impact of pregnancy test one day after implantation bleeding on fertility varies by individual factors including age, overall health, and specific medical history. Fertility specialists evaluate each case to optimize treatment protocols and maximize success rates.
Evaluation should be considered when experiencing fertility challenges, irregular symptoms, or concerns about reproductive health. Early consultation with fertility experts helps identify issues and implement timely interventions.
Treatment approaches for pregnancy test one day after implantation bleeding range from lifestyle modifications to advanced medical interventions. The optimal strategy depends on individual diagnosis, severity, and patient preferences discussed with healthcare providers.
Preparation involves comprehensive medical evaluation, following pre-treatment instructions, optimizing general health, and maintaining open communication with the healthcare team throughout the process.
ఆరోగ్యకరమైన జీవనశైలి అలవాట్లు సంతాన సామర్థ్యాన్ని పెంచుతాయి. సమతుల్య ఆహారం తినడం, ఆరోగ్యకరమైన బరువును కలిగి ఉండటం, క్రమం తప్పని వ్యాయామం, మద్యం మరియు కెఫిన్ వాడకాన్ని తగ్గించడం, ధూమపానం మానేయడం మరియు ఒత్తిడిని సమర్థవంతంగా నిర్వహించడం వంటివి ఇందులో ఉన్నాయి. ఈ మార్పులు హార్మోన్ల నియంత్రణకు మరియు ప్రజనన ఆరోగ్యానికి మద్దతు ఇస్తాయి.
బేసల్ బాడీ టెంపరేచర్ను ట్రాక్ చేయడం, ఓవ్యులేషన్ ప్రిడిక్టర్ కిట్స్ (OPKs) వాడటం, గర్భాశయ శ్లేష్మంలో మార్పులను గమనించడం లేదా ఫెర్టిలిటీ ట్రాకింగ్ యాప్లను ఉపయోగించడం వంటి అనేక పద్ధతుల ద్వారా మీరు అండోత్పత్తిని గుర్తించవచ్చు. గర్భధారణకు మీ అత్యంత సారవంతమైన రోజులను గుర్తించడానికి ఈ సాధనాలు సహాయపడతాయి.
అవును, అధిక ఒత్తిడి స్థాయిలు గర్భం దాల్చే మీ సామర్థ్యంపై ప్రభావం చూపుతాయి. ఒత్తిడి హార్మోన్ల సమతుల్యతను దెబ్బతీస్తుంది, మహిళల్లో అండోత్పత్తికి ఆటంకం కలిగిస్తుంది మరియు పురుషుల్లో వీర్యం నాణ్యతను తగ్గిస్తుంది. విశ్రాంతి పద్ధతులు, వ్యాయామం మరియు మానసిక మద్దతు ద్వారా ఒత్తిడిని నిర్వహించడం సంతాన సామర్థ్యాన్ని మెరుగుపరచడంలో సహాయపడుతుంది.
మీ వయస్సు 35 ఏళ్లలోపు ఉండి, 12 నెలల పాటు క్రమం తప్పకుండా, అసురక్షితంగా కలయికలో పాల్గొన్న తర్వాత కూడా గర్భం దాల్చకపోతే సంతాన సాఫల్య నిపుణులను సంప్రదించాలి. మీ వయస్సు 35 ఏళ్లు పైబడితే, ఆరు నెలల ప్రయత్నం తర్వాత సహాయం కోరండి. మీకు తెలిసిన ప్రజనన సమస్యలు లేదా క్రమం తప్పిన ఋతుచక్రాలు ఉంటే తక్షణమే వైద్యులను సంప్రదించడం మంచిది.
అవును, ఇది పూర్తిగా సాధారణం. చాలా ఆరోగ్యకరమైన జంటలు 6 నుండి 12 నెలలలోపు గర్భం దాలుస్తారు. మీ వయస్సు 35 ఏళ్లలోపు ఉండి, కేవలం మూడు నెలలుగా ప్రయత్నిస్తుంటే, సాధారణంగా ఆందోళన చెందాల్సిన అవసరం లేదు. సంతాన సాఫల్య పరీక్షలు చేయించుకోవడానికి ముందు వైద్యులు సాధారణంగా ఒక సంవత్సరం వరకు వేచి ఉండమని సిఫార్సు చేస్తారు.
You might start to feel subtle symptoms like mild cramping or spotting within a few days of the embryo transfer, around the time of implantation. However, many women feel nothing at all and still have a successful pregnancy. Its important not to worry if you dont notice any changes.
Yes, mild cramping is very normal after an embryo transfer. It can be a sign of the embryo implanting into the uterine wall or simply your uterus adjusting. These cramps are usually much lighter than period cramps.
Yes, absolutely. The progesterone supplements you take after the transfer are known to cause symptoms that are identical to early pregnancy signs, such as breast tenderness, bloating, fatigue, and mood swings. This is why the only reliable way to confirm a pregnancy is with a blood test.
- Severe abdominal pain
- Heavy bleeding (more than light spotting)
- Fever
- Signs of Ovarian Hyperstimulation Syndrome (OHSS), such as extreme bloating, rapid weight gain, or shortness of breath.
Early home pregnancy tests are not recommended after IVF. Testing too soon can lead to a false negative because the pregnancy hormone (hCG) might not be high enough to be detected yet. It can also lead to a false positive if you had an hCG “trigger shot,” as the hormone can remain in your system. The most accurate and reliable confirmation is the beta hCG blood test scheduled by your doctor.
After a failed implantation, your period will typically start on or around its expected date, which is usually 10 to 16 days after ovulation. Your body simply continues its normal menstrual cycle.
Yes, its possible for your period to be a few days late even if implantation did not happen. This slight delay can be caused by minor hormonal fluctuations from the conception attempt, stress, or changes in your routine.
Failed implantation itself does not cause spotting. However, the start of your period can begin with light spotting before the full flow arrives. This is different from “implantation spotting,” which is a sign of a successful implantation and happens much earlier.
The most reliable way to confirm if implantation has failed is by getting a negative pregnancy test on or after the day you expect your period.
Yes, if you suspect you are experiencing repeated implantation failures, especially after several months of trying to conceive or after IVF cycles, it is a good idea to consult a fertility specialist. Our team at Ferty9 can help investigate potential underlying causes and create a plan to improve your chances of success.
Yes, it’s possible to feel mild symptoms like cramping, spotting, or fatigue, but these can also be caused by the progesterone medication you are taking.
For many women, it feels like nothing at all. For others, it can feel like mild, short-lived cramping, a pulling sensation, or light twinges in the lower abdomen.
It is not recommended. A home pregnancy test at 7 days is likely to be negative even if you are pregnant because the hCG hormone levels are still too low to be detected in urine.
Not at all. Having no symptoms is very common and does not mean your IVF cycle has failed. Many women with successful pregnancies have no early signs.
Light pink or brown spotting that lasts for a day or two can be implantation bleeding. Heavy, red bleeding that progresses is more likely to be your period. If you are unsure, it’s always best to contact your doctor.
An HSG (Hysterosalpingography) test is a special X-ray procedure that uses a safe dye to get a clear picture of the inside of your uterus and fallopian tubes. It helps doctors see if your fallopian tubes are open.
Sedation is not usually needed for an HSG test. Most women experience mild to moderate cramping, similar to period pain, when the dye is injected. This discomfort is temporary and usually ends right after the procedure. Taking a simple painkiller beforehand can help.
Preparation is simple. You should inform your doctor of any allergies (especially to iodine), ensure you are not pregnant, and take any prescribed painkillers or antibiotics as advised.
The test must be done at a specific time in your menstrual cycle: after your period has finished but before you ovulate. This is usually between Day 6 and Day 10 of your cycle.
The HSG test is very safe. The most common side effects are mild and temporary, including cramping and light spotting for a day or two. There is a very small risk of infection or an allergic reaction to the dye.
Interestingly, yes. Some women become pregnant naturally in the months following an HSG test. It is believed that the pressure of the dye can sometimes help flush out minor blockages or mucus plugs in the fallopian tubes, increasing the chances of conception.
You will get your results very quickly. Your fertility specialist will typically discuss the findings with you immediately after the procedure is completed.
- Normal Result: The dye flows freely through the uterus and spills out of both fallopian tubes, meaning the tubes are open.
- Abnormal Result: The dye is blocked from spilling out, indicating a tubal blockage. The test can also reveal issues like fibroids or polyps inside the uterus.
It is recommended to abstain from any sexual activity, including ejaculation, for 2 to 5 days before your test. This ensures the sperm count and quality in your sample are at their optimal level for accurate analysis.
While some clinics may allow home collection in special cases, it is highly recommended to provide the sample at our Ferty9 centre in Hyderabad. The sample must be analyzed within 60 minutes of collection to ensure accuracy, and our private, comfortable rooms make the process discreet and convenient.
At Ferty9, we prioritize quick and clear results. You can typically expect to receive your semen analysis report on the same day, followed by a detailed consultation with one of our fertility specialists to discuss the findings.
Several factors can affect sperm quality, including lifestyle choices like smoking, excessive alcohol consumption, and stress. Other factors include poor diet, obesity, exposure to excessive heat (like from hot tubs or laptops on the lap), and certain medical conditions or medications.
Yes, in many cases, abnormal results can be improved. Depending on the cause, improvements can be made through lifestyle changes, dietary supplements, medications to balance hormones, or specific treatments for underlying conditions like varicocele.
No, the semen analysis test is completely painless. The collection process is non-invasive, and the analysis is done in the laboratory.
Because sperm quality can vary from day to day, your doctor will often recommend repeating the test after a few weeks to confirm the results. This ensures that any treatment plan is based on a consistent and accurate understanding of your fertility.
A standard semen analysis can suggest an infection if a high number of white blood cells are present. However, to confirm an infection, a separate test called a semen culture is required.
A result of zero sperm is called Azoospermia. This does not mean you can’t become a father. Our specialists at Ferty9 will recommend further tests to find the cause. In many cases, sperm can be successfully retrieved directly from the testicles using advanced procedures like TESA or Micro-TESE and used in IVF-ICSI.
సాధారణ నష్టాలలో ఒవేరియన్ హైపర్స్టిమ్యులేషన్ సిండ్రోమ్ (OHSS), బహుళ గర్భాలు, ఎక్టోపిక్ ప్రెగ్నెన్సీ, మరియు మందుల దుష్ప్రభావాలు ఉన్నాయి. చాలా సమస్యలు అరుదైనవి మరియు సరైన వైద్య పర్యవేక్షణ మరియు అధునాతన పర్యవేక్షణ పద్ధతులతో నిర్వహించదగినవి.
తయారీలో సమగ్ర సంతానోత్పత్తి పరీక్షలు, జీవనశైలి ఆప్టిమైజేషన్, పోషకాహార సప్లిమెంట్లు, మందుల ప్రణాళికలు, మరియు మానసిక కౌన్సెలింగ్ ఉంటాయి. చికిత్సకు ముందు మూల్యాంకనం చికిత్స విజయాన్ని ప్రభావితం చేసే కారకాలను గుర్తించి, పరిష్కరించడంలో సహాయపడుతుంది.
IVF విజయ శాతాలు వయస్సును బట్టి మారుతూ ఉంటాయి, 35 ఏళ్లలోపు మహిళలు అధిక విజయ శాతాలను (ఒక సైకిల్కు 40-50%) కలిగి ఉంటారు. విజయం అండం నాణ్యత, శుక్రకణ పారామితులు, గర్భాశయ ఆరోగ్యం, మరియు క్లినిక్ నైపుణ్యం వంటి కారకాలపై ఆధారపడి ఉంటుంది.
ఆదర్శ అభ్యర్థులలో ఫెలోపియన్ ట్యూబ్స్ మూసుకుపోయిన మహిళలు, ఎండోమెట్రియోసిస్, పురుష కారక సంతానలేమి, వివరించలేని సంతానలేమి, లేదా ఎక్కువ వయస్సు ఉన్నవారు ఉంటారు. సంతాన సాఫల్య నిపుణులు ప్రతి కేసును వైద్య చరిత్ర, రోగ నిర్ధారణ పరీక్షలు, మరియు చికిత్సా లక్ష్యాల ఆధారంగా వ్యక్తిగతంగా అంచనా వేస్తారు.
IVFలో అండాశయాల ఉత్తేజం, అండాల సేకరణ, ప్రయోగశాలలో ఫలదీకరణం, పిండం కల్చర్, మరియు బదిలీ ఉంటాయి. ఈ ప్రక్రియ సాధారణంగా 4-6 వారాలు పడుతుంది, ప్రతి దశలోనూ జాగ్రత్తగా పర్యవేక్షణ ఉంటుంది.
ఆధునిక వైద్య పురోగతులు, ముఖ్యంగా అసిస్టెడ్ రిప్రొడక్టివ్ టెక్నాలజీస్ (ART), స్త్రీపురుషుల సంతాన సామర్థ్యంపై గణనీయంగా ప్రభావం చూపాయి. ఇన్ విట్రో ఫెర్టిలైజేషన్ (IVF), ఇంట్రాసైటోప్లాస్మిక్ స్పెర్మ్ ఇంజెక్షన్ (ICSI), మరియు ప్రీఇంప్లాంటేషన్ జెనెటిక్ టెస్టింగ్ (PGT) వంటి పద్ధతులు వివిధ సంతానలేమి సవాళ్లను అధిగమించడానికి జంటలకు సహాయపడ్డాయి. అదనంగా, హార్మోన్ థెరపీలు, శస్త్రచికిత్సా విధానాలు, మరియు రోగ నిర్ధారణ సాధనాలలో పురోగతులు లింగాల మధ్య సంతానలేమి యొక్క అవగాహన మరియు చికిత్సను మెరుగుపరిచాయి.
కుటుంబ నియంత్రణ నిర్ణయాలలో సంతానోత్పత్తి రేట్లు ముఖ్యమైన పాత్ర పోషిస్తాయి. తగ్గుతున్న సంతానోత్పత్తి రేట్లు ఉన్న ప్రాంతాల్లో జంటలు తక్కువ మంది పిల్లలను కనాలని ఎంచుకోవచ్చు లేదా వయస్సు-సంబంధిత సంతానోత్పత్తి క్షీణత గురించిన ఆందోళనల కారణంగా పిల్లలను కనడాన్ని ఆలస్యం చేయవచ్చు. దీనికి విరుద్ధంగా, అధిక సంతానోత్పత్తి రేట్లు ఉన్న ప్రాంతాలలో, జనాభా పెరుగుదలను నిర్వహించడానికి మరియు పునరుత్పత్తి ఆరోగ్యాన్ని ప్రోత్సహించడానికి కుటుంబ నియంత్రణ వనరులు మరియు విద్యకు ప్రాప్యత కీలకం అవుతుంది.
సహజంగా సంతానోత్పత్తి రేట్లను మెరుగుపరచుకోవడానికి, సమతుల్య ఆహారం, క్రమం తప్పని వ్యాయామం, ఒత్తిడిని నిర్వహించడం, మరియు పొగాకు, అధిక మద్యపానం వంటి హానికరమైన పదార్థాలకు దూరంగా ఉండటం ద్వారా ఆరోగ్యకరమైన జీవనశైలిని అవలంబించండి. అదనంగా, అండం విడుదల చక్రాలను ట్రాక్ చేయడం మరియు దానికి అనుగుణంగా లైంగిక కలయికను సమయం చేసుకోవడం గర్భధారణ అవకాశాలను పెంచుతుంది.
భారతదేశంలో సాధారణ అపోహలు ఏమిటంటే, సంతానలేమి కేవలం మహిళల సమస్య అని, జీవనశైలి అలవాట్లు సంతానోత్పత్తిని ప్రభావితం చేయవని, లేదా ఒత్తిడే ప్రాథమిక కారణం అని నమ్మడం. వాస్తవానికి, స్త్రీపురుషులిద్దరూ సంతానోత్పత్తి సమస్యలను అనుభవించవచ్చు, మరియు ఆహారం, వ్యాయామం, ధూమపానం, మరియు మద్యం వంటి కారకాలు పెద్ద పాత్ర పోషిస్తాయి.
కాదు, సంతానలేమి కేవలం మహిళల సమస్య కాదు. పురుషులు కూడా సమానంగా ప్రభావితం కావచ్చు. తక్కువ శుక్రకణాల సంఖ్య లేదా తక్కువ శుక్రకణాల కదలిక వంటి పురుషుల సంతానలేమి, ఒక జంట గర్భం దాల్చే సామర్థ్యాన్ని గణనీయంగా ప్రభావితం చేస్తుంది. ఒక సంవత్సరం ప్రయత్నించిన తర్వాత గర్భం రాకపోతే ఇద్దరు భాగస్వాములు సంతానోత్పత్తి పరీక్షలు చేయించుకోవాలి.
జంటలు సంతాన సాఫల్య క్లినిక్లు, కౌన్సెలింగ్ సేవలు, ప్రభుత్వ పునరుత్పత్తి ఆరోగ్య కార్యక్రమాలు, మరియు IVF మరియు IUI వంటి సహాయక పునరుత్పత్తి సాంకేతికతల ద్వారా మద్దతు పొందవచ్చు. చాలా క్లినిక్లు వ్యక్తిగతీకరించిన చికిత్సా ప్రణాళికలు, భావోద్వేగ మద్దతు, మరియు సంతాన సాఫల్య నిపుణుల నుండి నిపుణుల మార్గదర్శకత్వం కూడా అందిస్తాయి.
ఆరోగ్యకరమైన జీవనశైలిని పాటించడం, అండం విడుదల (ఓవులేషన్)ను ట్రాక్ చేయడం, ఒత్తిడిని నిర్వహించడం, మరియు సకాలంలో వైద్య సలహా తీసుకోవడం వంటివి గర్భధారణ అవకాశాలను మెరుగుపరచడంలో సహాయపడతాయి. సమతుల్య ఆహారం, క్రమం తప్పని మోస్తరు వ్యాయామం, ధూమపానం మరియు అధిక మద్యపానానికి దూరంగా ఉండటం, మరియు ఆరోగ్యకరమైన బరువును కలిగి ఉండటం అన్నీ సంతానోత్పత్తికి ముఖ్యమైనవి.
6-12 నెలల పాటు గర్భధారణ ప్రయత్నాలు విఫలమైన తర్వాత పురుషులు మూల్యాంకనం చేయించుకోవాలి, లేదా సంతానోత్పత్తిని ప్రభావితం చేసే మునుపటి శస్త్రచికిత్సలు, ఇన్ఫెక్షన్లు, లేదా వైద్య పరిస్థితులు వంటి ప్రమాద కారకాలు ఉంటే వెంటనే చేయించుకోవాలి.
ఆహారం, వ్యాయామం, ధూమపానం, మద్యపానం, ఒత్తిడి స్థాయిలు, మరియు పర్యావరణ ప్రభావాలతో సహా జీవనశైలి కారకాలు శుక్రకణాల నాణ్యతను గణనీయంగా ప్రభావితం చేస్తాయి. ఈ కారకాలను మెరుగుపరచడం సహజంగా సంతానోత్పత్తి ఫలితాలను మెరుగుపరుస్తుంది.
జీవనశైలి మార్పులు మరియు మందుల నుండి శస్త్రచికిత్స జోక్యాలు మరియు సహాయక పునరుత్పత్తి పద్ధతుల వరకు చికిత్సలు ఉంటాయి. వెరికోసిల్ మరమ్మత్తు, హార్మోన్ థెరపీ, స్పెర్మ్ రిట్రీవల్ విధానాలు (PESA/TESA), మరియు తీవ్రమైన కేసులకు ICSI వంటివి ఎంపికలలో ఉన్నాయి.
మూల్యాంకనంలో వీర్య విశ్లేషణ (సెమెన్ ఎనాలిసిస్), హార్మోన్ల పరీక్ష, శారీరక పరీక్ష, జన్యుపరమైన పరీక్ష, మరియు అవసరమైతే ఇమేజింగ్ అధ్యయనాలు ఉంటాయి. వివరణాత్మక అంచనా నిర్దిష్ట కారణాలను గుర్తించడానికి మరియు తగిన చికిత్సా వ్యూహాలను మార్గనిర్దేశం చేయడానికి సహాయపడుతుంది.
సాధారణ కారణాలలో వెరికోసిల్, హార్మోన్ల అసమతుల్యతలు, జన్యుపరమైన కారకాలు, ఇన్ఫెక్షన్లు, జీవనశైలి కారకాలు, మరియు పర్యావరణ ప్రభావాలు ఉన్నాయి. సుమారు 40-50% సంతానలేమి కేసులలో పురుష కారకాలు ఉంటాయి, ఇది జంటలకు సమగ్ర మూల్యాంకనం అవసరమని సూచిస్తుంది.
Most clinics look for a minimum thickness of 7 mm before an embryo transfer. However, an ideal lining is often considered to be 8 mm or more, with a good “trilaminar” pattern.
In some cases, yes. If the thin lining is due to poor blood flow or lifestyle factors, changes in diet, exercise, and stress management can make a significant difference. If it is due to scarring or hormonal issues, medical treatment is usually necessary.
It typically takes the first half of your menstrual cycle (about 10-14 days) for the lining to grow. If you are on a medicated cycle for IVF, your doctor will monitor you with scans to track its growth over a similar period.
Yes, absolutely. Your body builds the uterine lining from the nutrients you provide it. A diet rich in vitamins, minerals, and compounds that improve blood flow (like L-arginine and Vitamin E) gives your body the tools it needs to build a healthy, thick lining.
PRP is considered a very safe procedure because it uses your body’s own concentrated platelets, so there is no risk of rejection or allergic reaction. For women with a persistently thin lining, studies have shown it to be an effective treatment for improving thickness and implantation rates.
The ideal age is in your late 20s or early 30s (before 35). This is when your egg quality and quantity are at their peak.
Thanks to vitrification, eggs can be stored for many years without any decline in quality. Legally, you can store them for up to 10 years, with extensions possible under certain conditions.
No, it is not a 100% guarantee. However, it significantly increases your chances of having a biological child later in life, especially when compared to trying with older eggs.
Yes, absolutely. The ART (Regulation) Act, 2021, allows single and unmarried women above the age of 21 to legally access ART services, including egg freezing, to preserve their fertility.
Yes, absolutely. Eating a small handful of mixed dry fruits daily provides essential vitamins and minerals needed for a healthy pregnancy.
The best time is usually in the morning, ideally on an empty stomach (especially soaked almonds and raisins). However, they also make for a great evening snack to curb hunger pangs.
Nutritionally, raw or soaked nuts are better because high heat can destroy some nutrients. However, lightly dry-roasted nuts are safe and can be easier to eat if you have nausea. Avoid oil-fried or salted versions.
In Indian tradition, some dry fruits like almonds, dates, and walnuts are considered “heat-producing” (Garam Taseer). Soaking them in water overnight effectively reduces this heat and makes them safe and easy to digest.
Dates (Khajoor), Raisins (Kishmish), Figs (Anjeer), and Apricots are excellent for boosting iron levels and hemoglobin.
Yes, they are safe. In fact, the folate in almonds and walnuts is crucial during the first trimester to prevent birth defects. However, if you suffer from severe nausea, eat them in small quantities.
తయారీలో సమగ్ర వైద్య మూల్యాంకనం, చికిత్సకు ముందు సూచనలను అనుసరించడం, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచుకోవడం, మరియు ప్రక్రియ అంతటా ఆరోగ్య బృందంతో బహిరంగ సంభాషణను నిర్వహించడం వంటివి ఉంటాయి.
ఒత్తిడి మరియు లైంగిక ఆరోగ్యంపై దాని ప్రభావాలకు చికిత్సా విధానాలు జీవనశైలి మార్పుల నుండి అధునాతన వైద్య జోక్యాల వరకు ఉంటాయి. ఉత్తమ వ్యూహం వ్యక్తిగత రోగ నిర్ధారణ, తీవ్రత, మరియు ఆరోగ్య సంరక్షణ ప్రదాతలతో చర్చించిన రోగి ప్రాధాన్యతలపై ఆధారపడి ఉంటుంది.
సంతానోత్పత్తి సవాళ్లు, అసాధారణ లక్షణాలు, లేదా పునరుత్పత్తి ఆరోగ్యం గురించి ఆందోళనలను ఎదుర్కొంటున్నప్పుడు మూల్యాంకనం పరిగణించాలి. సంతాన సాఫల్య నిపుణులతో ముందస్తు సంప్రదింపులు సమస్యలను గుర్తించడానికి మరియు సకాలంలో జోక్యాలను అమలు చేయడానికి సహాయపడతాయి.
సంతానోత్పత్తిపై ఒత్తిడి మరియు లైంగిక ఆరోగ్యంపై దాని ప్రభావాల ప్రభావం వయస్సు, మొత్తం ఆరోగ్యం, మరియు నిర్దిష్ట వైద్య చరిత్రతో సహా వ్యక్తిగత కారకాలచే మారుతుంది. సంతాన సాఫల్య నిపుణులు చికిత్సా ప్రణాళికలను ఆప్టిమైజ్ చేయడానికి మరియు విజయ శాతాలను గరిష్టంగా పెంచడానికి ప్రతి కేసును మూల్యాంకనం చేస్తారు.
సమాచారంతో కూడిన సంతానోత్పత్తి నిర్ణయాలు తీసుకోవడానికి ఒత్తిడి మరియు లైంగిక ఆరోగ్యంపై దాని ప్రభావాలను అర్థం చేసుకోవడం ముఖ్యం. ఈ పరిస్థితి/ప్రక్రియకు వ్యక్తిగత పరిస్థితుల ఆధారంగా తగిన చికిత్సా విధానాలను మరియు ఆశించిన ఫలితాలను నిర్ణయించడానికి సంతాన సాఫల్య నిపుణులచే సమగ్ర మూల్యాంకనం అవసరం.
సహజ ఆప్టిమైజేషన్లో ఆరోగ్యకరమైన బరువును నిర్వహించడం, సమతుల్య పోషణ, క్రమం తప్పని వ్యాయామం, ఒత్తిడి నిర్వహణ, హానికరమైన పదార్థాలకు దూరంగా ఉండటం, మరియు సరైన సమయం కోసం అండం విడుదల చక్రాలను ట్రాక్ చేయడం వంటివి ఉంటాయి.
వయస్సు అండం నాణ్యత మరియు పరిమాణాన్ని గణనీయంగా ప్రభావితం చేస్తుంది, 35 ఏళ్ల తర్వాత సంతానోత్పత్తి క్షీణిస్తుంది మరియు 40 తర్వాత మరింత వేగంగా తగ్గుతుంది. ముందస్తు జోక్యం మరియు సంతాన సామర్థ్య పరిరక్షణ ఎంపికలు వయస్సు-సంబంధిత సవాళ్లను పరిష్కరించడంలో సహాయపడతాయి.
చికిత్సలలో అండం విడుదల ప్రేరణ, శస్త్రచికిత్సా విధానాలు, జీవనశైలి మార్పులు, మరియు సహాయక పునరుత్పత్తి సాంకేతికతలు (ART) ఉంటాయి. చికిత్స ఎంపిక రోగ నిర్ధారణ, వయస్సు, మరియు వ్యక్తిగత పరిస్థితులపై ఆధారపడి ఉంటుంది.
అంచనాలో హార్మోన్ల పరీక్ష, అండాల నిల్వ మూల్యాంకనం (Ovarian reserve test), పెల్విక్ అల్ట్రాసౌండ్, హిస్టెరోసాల్పింగోగ్రామ్ (HSG), మరియు కొన్నిసార్లు లాపరోస్కోపీ ఉంటాయి. సమగ్ర పరీక్ష సంతానోత్పత్తిని ప్రభావితం చేసే నిర్దిష్ట కారకాలను గుర్తిస్తుంది.
ప్రధాన కారణాలలో అండం విడుదల లోపాలు, ఫెలోపియన్ ట్యూబ్స్ మూసుకుపోవడం, ఎండోమెట్రియోసిస్, గర్భాశయ అసాధారణతలు, వయస్సు-సంబంధిత కారకాలు, మరియు హార్మోన్ల అసమతుల్యతలు ఉంటాయి. ప్రతి పరిస్థితికి నిర్దిష్ట రోగ నిర్ధారణ విధానాలు మరియు చికిత్సా వ్యూహాలు అవసరం.
While millions are released, it takes only one sperm to penetrate and fertilize the egg. The millions act as support to help that one sperm reach the destination.
Yes. Pregnancy is possible with a low sperm count, though it may take longer naturally. Assisted reproductive technologies like IUI or ICSI can help achieve pregnancy even with very low counts.
A normal sperm count is defined as 15 million sperm per milliliter of semen or a total of 39 million sperm per ejaculation.
You can improve sperm health by quitting smoking, limiting alcohol, maintaining a healthy weight, reducing stress, and eating a diet rich in antioxidants, zinc, and vitamins.
Yes. While men produce sperm throughout their lives, sperm quality (motility and DNA integrity) tends to decline after age 40-45.
Foods rich in antioxidants are best. Include walnuts, citrus fruits, dark green leafy vegetables, eggs, and fish in your diet to boost sperm health.
No. Once periods have stopped for 12 months, the ovaries no longer release eggs, making natural conception impossible.
Success depends on endometrial response. Rates may be slightly lower for women close to age 50 due to health factors.
Yes. If you froze your eggs when you were younger, they can be thawed and fertilized for use after menopause (provided you are under the legal age limit of 50).
The best time to freeze eggs is before age 35 (ideally late 20s to early 30s) to ensure high quality.
The main risks include high blood pressure (preeclampsia), gestational diabetes, heart strain on the mother, and premature birth.
No. Hormone therapy can manage menopausal symptoms and prepare the womb for an IVF pregnancy, but it cannot make ovaries produce eggs again.
Adoption is a wonderful alternative. Under Indian law (CARA), prospective parents have specific age criteria, but adoption remains a viable path for many.
The average age for menopause in India is typically between 46 and 49 years, which is slightly earlier than the global average.
The earliest signs are usually irregular periods (perimenopause) and mood swings, followed by hot flashes.
No, menopause cannot be delayed naturally as it depends on your ovarian reserve (the number of eggs you have), which is determined by genetics and age.
Yes, hormonal changes slow down metabolism, leading to weight gain, particularly around the abdomen. Regular exercise and diet control can help.
Foods rich in calcium (dairy, leafy greens) and phytoestrogens (soy chunks, flaxseeds, chickpeas) can help balance hormones and protect bones.
This varies. For some, symptoms last 6 months to 2 years. For others, they may persist for 5 years or more, though the intensity usually decreases over time.
Hormone Replacement Therapy can have side effects and risks (like blood clots or breast cancer risk in some cases). It is only prescribed after a thorough medical evaluation.
You cannot get pregnant once menopause is confirmed (12 months without a period). However, you can get pregnant during perimenopause when periods are irregular.
With an impressive and consistent over 65% IVF success rate of Ferty9, the best IVF center in Vizag, is dedicated to providing couples with healthy and successful pregnancies.
In males, there can be certain reasons for infertility, like lower sperm count, terminal diseases, hormonal problems, or genetic disorders. On the other hand, in females, it can be ovulation disorders, fallopian tube damage, endometriosis, or terminal diseases.
At Ferty9 IVF center in Vizag, the treatment charges range from 1.5 to 2 lacs and usually depend on your health complexities and the underlying condition of your infertility. However, we always keep our IVF cost in Vizag transparent.
While choosing an IVF center, always look for:
- High success rates.
- Safety & Privacy
- Transparency throughout the treatment
- Ethics followed by the team
- Medical expertise.
- Infrastructure and equipment.
- Cost and financial considerations.
- Location and accessibility.
When you have infertility issues like fallopian tube damage, previous IUI failures, risk of genetic diseases, unexplained infertility, lower sperm counts, or pelvic adhesions, you should go straight for IVF treatment for better chances of pregnancy.
Ferty9 IVF center in Vijayawada has over 65% consistent success rates in IVF as IVF doctors in Vijayawada are committed to providing the best treatments, transparency, and care to couples.
The IVF cost in Vijayawada ranges from 1.5 to 2 lacs. However, the costs can differ depending on the underlying factors causing infertility, a patient’s age, and the health complexities of the patient. Ferty9 keeps the treatment charges transparent and affordable.
Yes, IVF babies are as normal as other children as far as their mental attributes and physical capabilities are concerned. Also, they are as naturally born as other children.
An individual is generally recommended by an infertility specialist to go for 1 to 3 IVF cycles. However, if none of them are successful, a fertility specialist might suggest more cycles.
At Ferty9’s IVF Centres in Rajahmundry, the first consultation with USG (Ultrasound Sonography) is completely free of charge.
Yes! You can get a free first consultation with an ultrasound scan at our best fertility hospital in Tirupati. You can book an appointment by calling 1800 296 0000.
Ideally, couples are advised to try to conceive naturally for at least a year before choosing IVF treatment. However, if a couple is over 35, they must consider a checkup from a fertility doctor after six months of trying to conceive.
The cost of IVF treatment in Tirupati ranges between Rs.1.5 and 2 Lacs, depending on the techniques used and the cause of infertility. However, Ferty9 ensures the most affordable treatment costs for patients.
At Ferty9 IVF hospital in Tirupati, patients can get their first consultation absolutely free of charge. Not only that, the first consultation also includes USG (Ultrasound Sonography). Call us toll-free at 1800 296 0000 to schedule your appointment.
IVF, established since the birth of Louise Brown in 1978, is a safe and effective fertility treatment. It has resulted in over 10 million births worldwide, demonstrating its long-term success and wide acceptance in reproductive medicine.
Yes, at Ferty9 Kurnool, we offer complimentary initial consultations with our fertility specialists. This isn’t just a standard meeting; it’s an opportunity for us to understand your unique story and cultural background. Our fertilityexpert team inKurnool will explain treatment options clearly and in a way that resonates with you, ensuring you feel comfortable and empowered throughout the process. Additionally, our first consultations include Ultrasonography (USG), emphasizing our thoroughness and dedication to your fertility journey.
Transparency is fundamental to our approach at Ferty9. During the consultation, we discuss all available options, including success rates and costs, so you can make well-informed decisions. This commitment to open communication and culturally sensitive care distinguishes us.
The cost of IVF in Ferty9 Kurnool typically ranges between ₹1.5 to ₹2 lakhs, depending on individual treatment needs and protocols tailored to each couple’s fertility challenges. We prioritize transparent and affordable pricing for all our fertility services, ensuring accessible options for our patients without compromising the quality.
Our goal is to provide curative treatments from initial consultations through state-of-the-art reproductive technologies like IVF. Our highly trained fertility specialists are driven to support you throughout your conception quest. Contact us (18002960000) to learn more about how we can assist you in achieving your dream of starting a family.
Ferty9 Kurnool provides an extensive array of diagnostic tests to assess various fertility concerns. These tests include detailed hormonal assessments, semen analysis, ultrasound scans, and other specialized evaluations. Each test is carefully selected to pinpoint the underlying causes of infertility, ensuring the best treatment plans and positive results.
Absolutely! Ferty9 offers advanced genetic tests like Preimplantation Genetic Testing (PGT) to analyze embryos for chromosomal abnormalities. This allows for selecting healthy embryos with the highest chance of successful implantation and a healthy baby. Understanding potential issues early on can promote a smoother, more worry-free pregnancy journey.
Ferty9 is the best IVF hospital in Hyderabad, focusing on expert care and compassionate support. We provide the most advanced fertility solutions tailored to every possible infertility issue a couple faces.
If you are selecting the best IVF hospital in Hyderabad, you should consider expertise, personalised care, a modern IVF approach, and success rates.
Ferty9 has a significant presence in Hyderabad, with established fertility centres in LB Nagar, Secunderabad, Banjara Hills and Kukatpally.
Ferty9 offers a cost range of 1.50 lacs to 2 lacs for treatment options like IUI or IVF. Additionally, The actual cost of IVF treatment varies based on patients and their infertility issues.
Fertility treatments are eligible for couples struggling to conceive due to infertility. At Ferty9’s infertility centres in Hyderabad, we assess every case sincerely and come up with a personalised solution for couples wanting successful conception.
At our fertility centres in Hyderabad, we consistently achieve high success rates of over 60% in IVF treatments.
We offer a consistent success rate of over 60% in IVF treatments. However, it can differ depending on factors like previous pregnancy, quality of sperm, and selected embryos.
Yes, we offer the best infertility treatment in Karimnagar for male infertility, including TESA, PESA, TESE, Micro TESE, ICSI, and PICSI. We leverage assessments like physical scans, scrotal scans, and sperm DNA fragmentation index tests to plan a precise treatment.
Yes, we do. At Ferty9 Infertility Hospital in Karimnagar, treatment options for fertility in women include IVF, IUI, ICSI, Genetic program, laparoscopy, and hysteroscopy.
IVF success rates are generally highest for women under 35. While the most success occurs between 20 and 30, good results are still possible in the early 30s. After 35, success rates decline steadily. Age is just one factor influencing IVF success. Overall health, egg quality, and the cause of infertility also play a significant role.
The IVF treatment costs at Ferty9 range from 1.5 to 2 lacs depending on factors like age, health complexities, and underlying conditions causing infertility. However, we always keep competitive and affordable fertility treatment costs.
Ferty9’s promise is to give best in class success rates ensuring safety, ethics, transparency & compassionate care.
At Ferty9, whether you are getting PGT-A (Preimplantation Genetic Testing for Aneuploidies), PGT-M (for monogenic/single gene disorders), or PGT-SR (for structural rearrangements), the treatment charges depend on the number of embryos to be scanned.
Yes, as technology is advancing nowadays, IVF can be successful on the first try. However, it usually depends on factors like a couple’s age, health condition, complexity, and fertility issues.
Most couples go through 2 to 3 IVF cycles before conception has taken place. In some cases, it can reach up to 6 IVF cycles as well.
With a consistency of over 60% success rate, the Ferty9 IVF clinic in Kukatpally has an impeccable record in fertility treatment.
The IVF success rate of at Ferty9 is consistently over 80%. However, it can differ depending on factors like age, underlying causes of infertility, and quality of sperm. Our fertility specialists in Warangal and team of embryologists ensure precise lab processes and an environment that elevates our success rates to the best.
Benefits that couples get from choosing Ferty9 Infertility Hospital in Warangal as their treatment facility:
- We offer the best-in-class success rates.
- We prioritize your safety
- We are committed to ethical and trusted practices
- We establish clear and transparent communication
- We provide compassionate care and counseling
- We provide you with affordable infertility treatment solutions
Yes, male infertility problems are as common as female. Yes, male infertility problems are as common as female. Infertility affects both genders equally: 33% male, 33% female, and 33% due to both or unexplained causes. This highlights that both male and female infertility are equally common, each accounting for about a third of all cases. The most common factors of infertility in males are STDs, lower sperm count, and hormonal issues. On the other hand, issues like ovulation disorder, damaged fallopian tubes, and irregular periods can cause infertility in females.
The cost of infertility treatment ranges from 1.5 to 2 lacs, as it depends on the underlying causes of the patient’s infertility and the techniques used in the cycle. However, Ferty9 provides the most affordable infertility treatment in Warangal.
Common infertility issues in females are PCOS (Polycystic Ovary Syndrome), problems in the menstrual cycle, endometriosis, infections, and structural problems of the reproductive system.
You can call the Ferty9 infertility hospital in LB Nagar at 1800 296 0000 between working hours to book an appointment with qualified fertility experts and get your assessment done.
At Ferty9 IVF clinic in LB Nagar, two of the best fertility specialists, embryologists, and several other professional members are present to provide your care.
Ferty9 Infertility Hospital in LB Nagar is open from 9 am to 6 pm on working days of the week.
Ferty9 has a track record of over 60% success rates, and it backs our commitment to providing the utmost care to couples seeking infertility treatment.
Getting to know if a fertility treatment is right for you or not includes several factors. Some of those factors include:
- You are over 35 and trying to get pregnant for at least 6 months.
- You are under 35 and trying to get pregnant for at least a year.
- You are trying to get pregnant, but you have irregular and painful periods.
- Your partner has infertility issues like lower sperm count, erectile dysfunction, or terminal diseases.
Ferty9 boasts a consistent IVF success rate of 60%+, a testament to their expertise in Assisted Reproductive Technology.
Yes, infertile men can get assisted reproductive technology (ART) based treatments like Testicular Sperm Aspiration (TESA), Percutaneous Epididymal Sperm Aspiration (PESA), Intracytoplasmic Sperm Injection (ICSI), and hormone therapy to regain their fertility.
To book an appointment with fertility experts, call Ferty9, the best IVF centre in Secunderabad at 1800 296 0000 between the day’s working hours (9 am to 6 pm).
Most health insurance doesn’t cover the expenses of IVF and other fertility treatments. However, some of them might cover the underlying condition causing your infertility.
The cost of IVF at Ferty9 Banjara Hills ranges from ₹1.5 lakhs to ₹2 lakhs. The final cost depends on various factors, such as the specific treatment plan, underlying causes of infertility, and additional services required. We strive to provide affordable treatment options without compromising on quality and care.
At Ferty9 fertility center in Banjara Hills, we provide extensive support services, including:
- Counseling Services: To help couples manage stress and psychosocial health during fertility treatment.
- Support Groups: Connecting you with other couples whose IVF is positive.
- Follow-up Care: Continuous support even after the treatment cycle is completed.
- We believe emotional and psychological support is as important as medical treatment to achieve successful outcomes.
- Contact us today to take the first step toward building your family.
Ferty9 utilizes a variety of revolutionary diagnostic tests to pinpoint the cause of infertility and guide treatment recommendations. These tests may include:
- Hormonal Evaluations: To check hormone levels and ovarian function.
- Semen Analysis: To assess sperm count and quality.
- Ultrasound Scans: To examine the reproductive organs.
- Hysterosalpingography (HSG): To check the condition of the fallopian tubes and uterus.
At Ferty9 Banjara Hills, we offer a wide range of fertility treatments, including:
- Male and Female Infertility Examinations
- Intrauterine Insemination (IUI)
- In Vitro Fertilization (IVF)
- Intracytoplasmic Sperm Injection (ICSI)
- Physiological Intracytoplasmic Sperm Injection (PICSI)
- Blastocyst Culture
- Genetic Testing
- Fertility Preservation
Each treatment plan is customized based on individual needs and medical history to ensure the best possible outcome.
Booking an appointment at Ferty9 Banjara Hills is simple. You can call our toll-free number at 18002960000 or visit our website to book an appointment online. Our team is always ready to assist you with any queries and help you find the best time for your visit.
ప్రమాదం చాలా తక్కువ. కొన్ని క్యాన్సర్లతో కొద్దిపాటి సంబంధం ఉన్నప్పటికీ, ఎండోమెట్రియోసిస్ నేరుగా క్యాన్సర్కు కారణం కాదు. ఈ సమస్య ఉన్న చాలా మంది మహిళలకు క్యాన్సర్ రాదు.
ఇది కొన్నిసార్లు నెలలు నిండకముందే ప్రసవం వంటి కొన్ని ప్రమాదాలను పెంచవచ్చు. అయితే, మీ డాక్టర్ పర్యవేక్షణలో ఉంటే, ఎండోమెట్రియోసిస్ ఉన్న చాలా మంది మహిళలు ఆరోగ్యకరమైన గర్భధారణను పొందుతారు.
అవును, కచ్చితంగా. చాలా మంది మహిళలు సహజంగానే గర్భం దాలుస్తారు. ఇతరులకు, సర్జరీ నుండి IVF వరకు ఉన్న చికిత్సలు గర్భం పొందడంలో అత్యంత ప్రభావవంతంగా సహాయపడతాయి.
ఏ ఆహారం దీనిని పూర్తిగా నయం చేయలేనప్పటికీ, వాపును తగ్గించే (Anti-inflammatory) ఆహారం లక్షణాలను నియంత్రించడంలో సహాయపడుతుంది. దీని అర్థం పండ్లు, కూరగాయలు మరియు ఆరోగ్యకరమైన కొవ్వులు ఎక్కువగా తినడం మరియు ప్రాసెస్ చేసిన ఆహారం, పంచదార మరియు రెడ్ మీట్ (మటన్ వంటివి) తగ్గించడం.
The key difference is genetics. In gestational surrogacy (the only legal form in India), the surrogate has no genetic link to the baby. She carries an embryo created using the intended parents’ (or donors’) egg and sperm. In traditional surrogacy, the surrogate’s own egg is used, making her the biological mother—this practice is banned in India to protect legal parentage rights.
Yes, in most cases. The embryo is created using your egg and your partner’s sperm, so the child is 100% genetically yours.
- Update (March 2024): If one partner has a certified medical condition preventing them from providing gametes, the Supreme Court now allows the use of a donor egg or sperm (but not both; at least one gamete must come from the intending couple).
- Single Women (Widow/Divorcee): Must use their own eggs and donor sperm.
Under the Surrogacy (Regulation) Amendment Rules, 2023, a surrogate must be:
- A married woman (aged 25–35) with at least one child of her own.
- Physically and psychologically fit.
- A “willing woman” (she no longer needs to be a “close relative,” allowing friends or extended family to help).
- She can act as a surrogate only once in her lifetime.
Success depends heavily on the quality of the egg and sperm.
- Self-Cycle (Your Eggs <35): ~55–60% per transfer.
- Donor Cycle: ~75–95% success rate, as eggs from young, healthy donors are used.
- Cumulative Success: 90%+ couples achieve pregnancy within 3 attempts.
The cost typically ranges from ₹15 Lakhs to ₹20 Lakhs. Since commercial surrogacy is banned, this covers:
- Medical procedures (IVF, delivery, hospitalization).
- Surrogates insurance and care.
- Legal and administrative fees.
Ideally, yes. Indian law prioritizes the child having a genetic link to the intended parents. However, recent legal changes allow flexibility if there is a medical necessity.
Yes, but with conditions. As per the March 2024 Supreme Court amendment, you can use donor eggs only if the intended mother has a medical condition (like absent uterus or premature ovarian failure) certified by the District Medical Board. The husband’s sperm must be used to ensure a genetic link.
Yes, Altruistic Gestational Surrogacy is legal and strictly regulated under the Surrogacy (Regulation) Act, 2021. Commercial surrogacy (paying a surrogate for profit) is illegal.
It is common not to succeed on the first try. We typically create multiple embryos in one IVF cycle. If the first transfer fails, we can perform a Frozen Embryo Transfer (FET) in the next cycle without restarting the whole IVF process. A surrogate can undergo up to 3 embryo transfer attempts.
Yes, intended parents are usually present at the hospital for the birth to bond with the baby immediately, subject to hospital policy and the surrogate’s comfort.
The surrogate is covered by a mandatory 36-month health insurance policy purchased by the intended parents. This covers all postpartum complications and medical treatments required for her recovery.
Yes. Research shows that children born via surrogacy are as healthy as those born via natural conception or standard IVF. The surrogate undergoes rigorous infectious disease screening (HIV, Hep B/C, etc.) to ensure the babys safety.
The birth certificate will bear the names of the Intended Parents (you and your spouse). The surrogate’s name is not mentioned on the birth certificate, ensuring your legal parentage is clear from day one.
It is a legal order passed by a First Class Magistrate court after the birth. It formally establishes you as the legal parents and confirms that the surrogate has no parental rights or obligations toward the child.
Currently, No. Indian law restricts surrogacy to married heterosexual couples and specific categories of single women (widows/divorcees).
- Single Women: Yes, if she is a widow or divorcee between the ages of 35 and 45.
- Single Men: No, single men are not currently eligible under the Act.
The intended parents must provide health insurance coverage for the surrogate for a period of 36 months (3 years). This ensures she is protected long after the delivery.
This is legally protected. The surrogate signs a binding agreement before the cycle starts, relinquishing all rights. Under the Surrogacy (Regulation) Act, 2021, the child is deemed the biological child of the intended parents from birth, and the surrogate has no legal claim to custody.
Yes, since altruistic surrogacy often involves a willing woman or relative, many families choose to stay in touch. However, clear boundaries are established during counseling to ensure everyone’s emotional well-being.
Mandatory psychological counseling is provided for both the surrogate and the intended parents to prepare for the emotional journey, ensure informed consent, and prevent postpartum depression.
In the altruistic model, parents often find a surrogate from their own network (friends/family). If you cannot find one, Ferty9 can assist in connecting you with a willing candidate who meets all legal and medical criteria. Mutual compatibility is verified before proceeding.
Since you pay only for medical and legal costs (not a surrogate fee), payments are made in installments at specific milestones (e.g., registration, IVF start, pregnancy confirmation). Ferty9 offers EMI options and financing assistance to make the process manageable.
Yes. Non-Resident Indians (NRIs) and Overseas Citizens of India (OCI) cardholders are eligible for surrogacy in India, provided they are a legally married couple and meet the age/medical criteria.
Foreign citizens (without OCI status) are not eligible for surrogacy in India. The law strictly limits these services to Indian citizens and OCI holders to prevent commercial exploitation.
Yes, Altruistic Surrogacy is the only legal form of surrogacy in India under the Surrogacy (Regulation) Act, 2021. Commercial surrogacy is strictly banned.
Yes, recent amendments allow any “willing woman” who meets the eligibility criteria (age, married, has a child, medical fitness) to act as a surrogate. She does not necessarily have to be a close relative.
Parents must cover all medical expenses related to the pregnancy, delivery charges, postpartum care, and mandatory health insurance for 36 months. They cannot pay a salary or convenience fee.
It can be complex. The surrogate must be prepared to give the baby up, and parents must trust another woman with their child. However, with proper counselling and clear boundaries, it is a deeply rewarding experience.
Yes. You need a Certificate of Medical Indication (proving you need surrogacy medically) and a Certificate of Essentiality from the appropriate government authority before treatment can begin.
అవును, కడుపు నొప్పి ఇంప్లాంటేషన్కు ప్రారంభ సంకేతం కావచ్చు. ఇది సాధారణంగా ఫలదీకరణ జరిగిన 7 నుండి 10 రోజుల తర్వాత సంభవిస్తుంది మరియు తేలికపాటి నెలసరి నొప్పుల మాదిరిగానే అనిపిస్తుంది.
రక్త పరీక్ష చాలా తక్కువ స్థాయిలలో ఉన్న HCGని కూడా గుర్తించగలదు మరియు ఇది మూత్ర పరీక్ష కంటే సున్నితమైనది. ఇది గర్భధారణ జరిగిన 7 నుండి 10 రోజులలోనే గర్భాన్ని నిర్ధారించగలదు.
ఇంప్లాంటేషన్ బ్లీడింగ్ సాధారణంగా లేత గులాబీ లేదా గోధుమ రంగులో ఉంటుంది మరియు సాధారణ పీరియడ్ కంటే చాలా తక్కువగా ఉంటుంది. నెలసరి రక్తస్రావం తేలికగా మొదలై ముదురు ఎరుపు ప్రవాహంతో ఎక్కువవుతుంది, కానీ ఇంప్లాంటేషన్ బ్లీడింగ్ సమయం గడిచేకొద్దీ ఎక్కువ కాదు.
ఇంప్లాంటేషన్ విజయవంతమైతే, పిండ బదిలీ జరిగిన సుమారు రెండు వారాల తర్వాత ప్రారంభ గర్భధారణ లక్షణాలు సాధారణంగా కనిపిస్తాయి.
తయారీలో సమగ్ర వైద్య మూల్యాంకనం, చికిత్సకు ముందు సూచనలను అనుసరించడం, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచుకోవడం, మరియు ప్రక్రియ అంతటా ఆరోగ్య బృందంతో బహిరంగ సంభాషణను నిర్వహించడం వంటివి ఉంటాయి.
ముందస్తు మెనోపాజ్ తర్వాత గర్భం పొందడానికి చికిత్సా విధానాలు జీవనశైలి మార్పుల నుండి అధునాతన వైద్య జోక్యాల వరకు ఉంటాయి. ఉత్తమ వ్యూహం వ్యక్తిగత రోగ నిర్ధారణ, తీవ్రత, మరియు ఆరోగ్య సంరక్షణ ప్రదాతలతో చర్చించిన రోగి ప్రాధాన్యతలపై ఆధారపడి ఉంటుంది.
సంతానోత్పత్తి సవాళ్లు, అసాధారణ లక్షణాలు, లేదా పునరుత్పత్తి ఆరోగ్యం గురించి ఆందోళనలను ఎదుర్కొంటున్నప్పుడు మూల్యాంకనం పరిగణించాలి. సంతాన సాఫల్య నిపుణులతో ముందస్తు సంప్రదింపులు సమస్యలను గుర్తించడానికి మరియు సకాలంలో జోక్యాలను అమలు చేయడానికి సహాయపడతాయి.
ముందస్తు మెనోపాజ్ తర్వాత గర్భం పొందడం యొక్క ప్రభావం వయస్సు, మొత్తం ఆరోగ్యం, మరియు నిర్దిష్ట వైద్య చరిత్రతో సహా వ్యక్తిగత కారకాలచే మారుతుంది. సంతాన సాఫల్య నిపుణులు చికిత్సా ప్రణాళికలను ఆప్టిమైజ్ చేయడానికి మరియు విజయ శాతాలను గరిష్టంగా పెంచడానికి ప్రతి కేసును మూల్యాంకనం చేస్తారు.
సమాచారంతో కూడిన సంతానోత్పత్తి నిర్ణయాలు తీసుకోవడానికి ముందస్తు మెనోపాజ్ తర్వాత గర్భం పొందడం గురించి అర్థం చేసుకోవడం ముఖ్యం. ఈ పరిస్థితి/ప్రక్రియకు వ్యక్తిగత పరిస్థితుల ఆధారంగా తగిన చికిత్సా విధానాలను మరియు ఆశించిన ఫలితాలను నిర్ణయించడానికి సంతాన సాఫల్య నిపుణులచే సమగ్ర మూల్యాంకనం అవసరం.
తయారీలో సమగ్ర వైద్య మూల్యాంకనం, చికిత్సకు ముందు సూచనలను అనుసరించడం, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచుకోవడం, మరియు ప్రక్రియ అంతటా ఆరోగ్య బృందంతో బహిరంగ సంభాషణను నిర్వహించడం వంటివి ఉంటాయి.
యాంటిడిప్రెసెంట్స్ మరియు సంతానోత్పత్తిపై దాని ప్రభావాలకు చికిత్సా విధానాలు జీవనశైలి మార్పుల నుండి అధునాతన వైద్య జోక్యాల వరకు ఉంటాయి. ఉత్తమ వ్యూహం వ్యక్తిగత రోగ నిర్ధారణ, తీవ్రత, మరియు ఆరోగ్య సంరక్షణ ప్రదాతలతో చర్చించిన రోగి ప్రాధాన్యతలపై ఆధారపడి ఉంటుంది.
సంతానోత్పత్తి సవాళ్లు, అసాధారణ లక్షణాలు, లేదా పునరుత్పత్తి ఆరోగ్యం గురించి ఆందోళనలను ఎదుర్కొంటున్నప్పుడు మూల్యాంకనం పరిగణించాలి. సంతాన సాఫల్య నిపుణులతో ముందస్తు సంప్రదింపులు సమస్యలను గుర్తించడానికి మరియు సకాలంలో జోక్యాలను అమలు చేయడానికి సహాయపడతాయి.
సంతానోత్పత్తిపై యాంటిడిప్రెసెంట్స్ ప్రభావం వయస్సు, మొత్తం ఆరోగ్యం, మరియు నిర్దిష్ట వైద్య చరిత్రతో సహా వ్యక్తిగత కారకాలచే మారుతుంది. సంతాన సాఫల్య నిపుణులు చికిత్సా ప్రణాళికలను ఆప్టిమైజ్ చేయడానికి మరియు విజయ శాతాలను గరిష్టంగా పెంచడానికి ప్రతి కేసును మూల్యాంకనం చేస్తారు.
సమాచారంతో కూడిన సంతానోత్పత్తి నిర్ణయాలు తీసుకోవడానికి యాంటిడిప్రెసెంట్స్ మరియు సంతానోత్పత్తిపై దాని ప్రభావాలను అర్థం చేసుకోవడం ముఖ్యం. ఈ పరిస్థితి/ప్రక్రియకు వ్యక్తిగత పరిస్థితుల ఆధారంగా తగిన చికిత్సా విధానాలను మరియు ఆశించిన ఫలితాలను నిర్ణయించడానికి సంతాన సాఫల్య నిపుణులచే సమగ్ర మూల్యాంకనం అవసరం.
సిద్ధం కావడంలో సమగ్ర వైద్య పరీక్షలు చేయించుకోవడం, చికిత్సకు ముందు సూచనలను పాటించడం, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచుకోవడం మరియు ప్రక్రియ అంతటా వైద్య బృందంతో ఎప్పటికప్పుడు మాట్లాడటం ఉంటాయి.
సహజంగా అండం విడుదలను పెంచుకోవడానికి చికిత్సా విధానాలు జీవనశైలి మార్పుల నుండి అధునాతన వైద్య చికిత్సల వరకు ఉంటాయి. సరైన వ్యూహం అనేది వ్యక్తిగత రోగ నిర్ధారణ (Diagnosis), సమస్య తీవ్రత మరియు వైద్యులతో చర్చించిన రోగి ప్రాధాన్యతలపై ఆధారపడి ఉంటుంది.
సంతానోత్పత్తి సవాళ్లు, అసాధారణ లక్షణాలు లేదా పునరుత్పత్తి ఆరోగ్యం గురించి ఆందోళనలు ఉన్నప్పుడు మూల్యాంకనం గురించి ఆలోచించాలి. ఫెర్టిలిటీ నిపుణులతో ముందస్తు సంప్రదింపులు సమస్యలను గుర్తించడానికి మరియు సకాలంలో చికిత్సలు తీసుకోవడానికి సహాయపడతాయి.
సంతానోత్పత్తిపై ఈ చిట్కాల ప్రభావం వయస్సు, మొత్తం ఆరోగ్యం మరియు నిర్దిష్ట వైద్య చరిత్రతో సహా వ్యక్తిగత కారకాలపై మారుతుంది. చికిత్సా పద్ధతులను ఆప్టిమైజ్ చేయడానికి మరియు విజయ శాతాలను గరిష్టంగా పెంచడానికి ఫెర్టిలిటీ నిపుణులు ప్రతి కేసును అంచనా వేస్తారు.
సమాచారంతో కూడిన సంతానోత్పత్తి నిర్ణయాలు తీసుకోవడానికి ఈ చిట్కాలను అర్థం చేసుకోవడం ముఖ్యం. వ్యక్తిగత పరిస్థితుల ఆధారంగా తగిన చికిత్సా విధానాలను మరియు ఆశించిన ఫలితాలను నిర్ణయించడానికి ఫెర్టిలిటీ నిపుణుల సమగ్ర మూల్యాంకనం అవసరం.
6-12 నెలల పాటు గర్భధారణ ప్రయత్నాలు విఫలమైన తర్వాత పురుషులు మూల్యాంకనం చేయించుకోవాలి, లేదా సంతానోత్పత్తిని ప్రభావితం చేసే మునుపటి శస్త్రచికిత్సలు, ఇన్ఫెక్షన్లు, లేదా వైద్య పరిస్థితులు వంటి ప్రమాద కారకాలు ఉంటే వెంటనే చేయించుకోవాలి.
ఆహారం, వ్యాయామం, ధూమపానం, మద్యపానం, ఒత్తిడి స్థాయిలు, మరియు పర్యావరణ ప్రభావాలతో సహా జీవనశైలి కారకాలు శుక్రకణాల నాణ్యతను గణనీయంగా ప్రభావితం చేస్తాయి. ఈ కారకాలను ఆప్టిమైజ్ చేయడం సహజంగా సంతానోత్పత్తి ఫలితాలను మెరుగుపరుస్తుంది.
జీవనశైలి మార్పులు మరియు మందుల నుండి శస్త్రచికిత్స జోక్యాలు మరియు సహాయక పునరుత్పత్తి పద్ధతుల వరకు చికిత్సలు ఉంటాయి. వెరికోసిల్ రిపేర్, హార్మోన్ థెరపీ, స్పెర్మ్ రిట్రీవల్ విధానాలు, మరియు తీవ్రమైన కేసులకు ICSI వంటివి ఎంపికలలో ఉన్నాయి.
మూల్యాంకనంలో వీర్య విశ్లేషణ (సెమెన్ ఎనాలిసిస్), హార్మోన్ల పరీక్ష, శారీరక పరీక్ష, జన్యుపరమైన పరీక్ష, మరియు అవసరమైతే ఇమేజింగ్ అధ్యయనాలు ఉంటాయి. వివరణాత్మక అంచనా నిర్దిష్ట కారణాలను గుర్తించడానికి మరియు తగిన చికిత్సా వ్యూహాలను మార్గనిర్దేశం చేయడానికి సహాయపడుతుంది.
సాధారణ కారణాలలో వెరికోసిల్, హార్మోన్ల అసమతుల్యతలు, జన్యుపరమైన కారకాలు, ఇన్ఫెక్షన్లు, జీవనశైలి కారకాలు, మరియు పర్యావరణ ప్రభావాలు ఉన్నాయి. సుమారు 40-50% సంతానలేమి కేసులలో పురుష కారకాలు ఉంటాయి, ఇది జంటలకు సమగ్ర మూల్యాంకనం అవసరమని సూచిస్తుంది.
తయారీలో సమగ్ర వైద్య మూల్యాంకనం, చికిత్సకు ముందు సూచనలను అనుసరించడం, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచుకోవడం, మరియు ప్రక్రియ అంతటా ఆరోగ్య బృందంతో బహిరంగ సంభాషణను నిర్వహించడం వంటివి ఉంటాయి.
సంగీతం పిండం ఇంప్లాంటేషన్ను మెరుగుపరచగలదా అనే దానికి చికిత్సా విధానాలు జీవనశైలి మార్పుల నుండి అధునాతన వైద్య జోక్యాల వరకు ఉంటాయి. ఉత్తమ వ్యూహం వ్యక్తిగత రోగ నిర్ధారణ, తీవ్రత, మరియు ఆరోగ్య సంరక్షణ ప్రదాతలతో చర్చించిన రోగి ప్రాధాన్యతలపై ఆధారపడి ఉంటుంది.
సంతానోత్పత్తి సవాళ్లు, అసాధారణ లక్షణాలు, లేదా పునరుత్పత్తి ఆరోగ్యం గురించి ఆందోళనలను ఎదుర్కొంటున్నప్పుడు మూల్యాంకనం పరిగణించాలి. సంతాన సాఫల్య నిపుణులతో ముందస్తు సంప్రదింపులు సమస్యలను గుర్తించడానికి మరియు సకాలంలో జోక్యాలను అమలు చేయడానికి సహాయపడతాయి.
సంతానోత్పత్తిపై సంగీతం పిండం ఇంప్లాంటేషన్ను మెరుగుపరచగలదా అనే దాని ప్రభావం వయస్సు, మొత్తం ఆరోగ్యం, మరియు నిర్దిష్ట వైద్య చరిత్రతో సహా వ్యక్తిగత కారకాలచే మారుతుంది. సంతాన సాఫల్య నిపుణులు చికిత్సా ప్రణాళికలను ఆప్టిమైజ్ చేయడానికి మరియు విజయ శాతాలను గరిష్టంగా పెంచడానికి ప్రతి కేసును మూల్యాంకనం చేస్తారు.
సమాచారంతో కూడిన సంతానోత్పత్తి నిర్ణయాలు తీసుకోవడానికి సంగీతం పిండం ఇంప్లాంటేషన్ను మెరుగుపరచగలదా అనే దాని గురించి అర్థం చేసుకోవడం ముఖ్యం. ఈ పరిస్థితి/ప్రక్రియకు వ్యక్తిగత పరిస్థితుల ఆధారంగా తగిన చికిత్సా విధానాలను మరియు ఆశించిన ఫలితాలను నిర్ణయించడానికి సంతాన సాఫల్య నిపుణులచే సమగ్ర మూల్యాంకనం అవసరం.
మీ పీరియడ్స్ అస్తవ్యస్తంగా ఉంటే (Irregular periods) డాక్టర్ను సంప్రదించండి. ఇది మిమ్మల్ని ఇబ్బంది పెట్టకపోయినా, దీనికి గల కారణాన్ని మరియు సంభావ్య ఆరోగ్య ప్రమాదాలను పరిశీలించడం చాలా ముఖ్యం. మీ నెలసరి ఆలస్యమైతే లేదా ఆగిపోతే, మీరు ప్రెగ్నెన్సీ టెస్ట్ చేయించుకోవాలి.
తగినంత ప్రోటీన్, మొక్కల ఆధారిత కొవ్వులు, మరియు ఫైబర్ అధికంగా ఉండే కార్బోహైడ్రేట్లు మీ శరీరానికి సరైన పరిమాణంలో హార్మోన్లను ఉత్పత్తి చేయడానికి అవసరమైన వనరులను అందిస్తాయి. తగినంత నీరు తాగడం, ఐరన్ అధికంగా ఉండే ఆహారాలు, ఒమేగా-3 ఫిష్ ఆయిల్, మరియు అల్లం, దాల్చిన చెక్క వంటి కొన్ని మూలికలు నెలసరి నొప్పులు, కడుపు ఉబ్బరం, డిప్రెషన్, మరియు మూడ్ స్వింగ్స్ నుండి ఉపశమనం పొందడంలో సహాయపడతాయి.
ముఖ్యమైన కారణాల వల్ల మీ జీవితంలో మీ నెలసరి చక్రం గణనీయంగా మారుతుంది. కౌమారదశలో రజస్వల (Puberty) సంభవిస్తుంది, ఆ తర్వాత మీ అత్యంత ఫలవంతమైన సంవత్సరాలు (Most fertile years) టీనేజ్ చివరిలో మొదలై 20ల ప్రారంభంలో ముగుస్తాయి. మీ నెలసరి చక్రం టీనేజ్ నుండి పెరిమెనోపాజ్ మరియు మెనోపాజ్ వరకు దశాబ్దం నుండి దశాబ్దానికి మారవచ్చు.
శరీరం యొక్క ఒత్తిడి ప్రతిస్పందనలో హార్మోన్ల హెచ్చుతగ్గులు ఒక భాగం. మీ హార్మోన్లు ఎక్కువ కాలం అసమతుల్యంగా ఉన్నప్పుడు, పునరుత్పత్తి వంటి హార్మోన్ల ద్వారా నడిచే వ్యవస్థలు తరచుగా ప్రభావితమవుతాయి.
మీకు సాధారణంగా ఎలా ఉంటుందో చూడటానికి క్యాలెండర్పై మీ నెలసరి చక్రాన్ని ట్రాక్ చేయడం ప్రారంభించండి. మీ పీరియడ్స్ ఫ్రీక్వెన్సీని నిర్ణయించడానికి ప్రతి నెలా మీ ప్రారంభ తేదీని నోట్ చేసుకోవడంతో మొదలుపెట్టండి. మీ నెలసరి చక్రాన్ని ట్రాక్ చేయడానికి అనేక ఆన్లైన్ యాప్స్ కూడా అందుబాటులో ఉన్నాయి.
ఓవులేషన్ లక్షణాలలో సెర్వికల్ మ్యూకస్లో మార్పులు, రొమ్ముల సున్నితత్వం, పొత్తికడుపు నొప్పి, తేలికపాటి స్పాటింగ్ లేదా స్రావం, బేసల్ బాడీ టెంపరేచర్లో మార్పు, లైంగిక వాంఛలో మార్పులు, తలనొప్పి, మరియు కొన్నిసార్లు వికారం ఉంటాయి.
అవును, ఒత్తిడి మరియు ఇతర జీవనశైలి కారకాలు శరీరం విటమిన్లను ఎంత ప్రభావవంతంగా గ్రహిస్తుందో మరియు ఉపయోగిస్తుందో ప్రభావితం చేయగలవు. అధిక ఒత్తిడి స్థాయిలు హార్మోన్ల సమతుల్యతకు ఆటంకం కలిగిస్తాయి మరియు సంతానోత్పత్తికి మద్దతు ఇచ్చే పోషకాల సామర్థ్యాన్ని తగ్గిస్తాయి. సరైన పోషకాహారంతో పాటు ఒత్తిడి నిర్వహణ పద్ధతులను చేర్చడం సంతానోత్పత్తి ఫలితాలను మెరుగుపరుస్తుంది.
చాలా విటమిన్లు సంతానోత్పత్తికి మద్దతు ఇస్తుండగా, విటమిన్ A వంటి కొన్ని విటమిన్లను అధికంగా తీసుకోవడం పునరుత్పత్తి ఆరోగ్యాన్ని ప్రతికూలంగా ప్రభావితం చేస్తుంది. అధిక మోతాదులు టాక్సిసిటీ మరియు సమస్యలకు దారితీయవచ్చు. ఎల్లప్పుడూ సిఫార్సు చేయబడిన మోతాదులను పాటించండి మరియు సురక్షితమైన సప్లిమెంటేషన్ కోసం ఆరోగ్య సంరక్షణ ప్రదాతను సంప్రదించండి.
మెగ్నీషియం హార్మోన్ల నియంత్రణ మరియు పునరుత్పత్తి ఆరోగ్యానికి మద్దతు ఇస్తుంది. ఇది మహిళలలో ఓవులేషన్ మరియు పురుషులలో శుక్రకణాల ఉత్పత్తికి కీలకమైన వివిధ జీవరసాయన ప్రతిచర్యలలో పాల్గొంటుంది. తగినంత మెగ్నీషియం స్థాయిలు గర్భధారణకు మరింత అనుకూలమైన వాతావరణాన్ని సృష్టించడానికి సహాయపడతాయి.
అవును, గర్భం కోసం ప్రయత్నిస్తున్నప్పుడు విటమిన్ సప్లిమెంట్లను తీసుకోవడం సురక్షితమే. అయితే, మీ నిర్దిష్ట అవసరాలకు సరైన రకాలు మరియు మోతాదులను నిర్ణయించడానికి మీ ఆరోగ్య సంరక్షణ ప్రదాతను సంప్రదించడం ముఖ్యం. వ్యక్తిగత మార్గదర్శకత్వం మీరు మీ పునరుత్పత్తి ఆరోగ్యానికి ప్రభావవంతంగా మరియు సురక్షితంగా మద్దతు ఇస్తున్నారని నిర్ధారిస్తుంది.
బొప్పాయి, అనాసపండు (pineapple), మరియు అల్లం వంటి ఆహారాలు గర్భాశయ సంకోచాలను ప్రేరేపించి పీరియడ్స్ను కొద్దిగా ముందుకు తీసుకురావచ్చు. అయితే, దీనిని ప్రయత్నించే ముందు ఆరోగ్య సంరక్షణ ప్రదాతను సంప్రదించండి.
ఫలితాలు వ్యక్తిని బట్టి మారుతూ ఉంటాయి. యాంటీ-ఇన్ఫ్లమేటరీ ఆహారాలు అధికంగా ఉండే డైట్ను స్థిరంగా తీసుకోవడం వల్ల కొన్ని నెలసరి చక్రాల తర్వాత నొప్పులు తగ్గవచ్చు.
అవును, చక్కెర, కెఫిన్ మరియు అనారోగ్యకరమైన కొవ్వులు ఎక్కువగా ఉండే ఆహారాలు వాపును పెంచి నొప్పులను మరింత తీవ్రతరం చేస్తాయి. మెరుగైన నొప్పి నివారణ కోసం ప్రాసెస్ చేసిన మరియు వేయించిన ఆహారాలను నివారించండి.
అవును. వయస్సుతో పాటు అండం నాణ్యత తగ్గుతుంది కాబట్టి, చిన్న వయస్సులోనే అండాలను గడ్డకట్టించడం (Freezing) ద్వారా మహిళలు తమ పునరుత్పత్తి సామర్థ్యాన్ని కాపాడుకోవచ్చు. భవిష్యత్తులో సహజ సంతానోత్పత్తి తగ్గినప్పుడు ఈ ఆరోగ్యకరమైన అండాలను ఉపయోగించుకోవచ్చు.
అవును. CoQ10, DHEA, ఫోలిక్ యాసిడ్ మరియు యాంటీఆక్సిడెంట్లు వంటి సప్లిమెంట్లు అండాల ఆరోగ్యాన్ని ప్రోత్సహించడానికి మరియు నాణ్యతను మెరుగుపరచడానికి సిఫార్సు చేయబడతాయి. ఇవి కణాలకు శక్తిని ఇవ్వడానికి మరియు నష్టం నుండి కాపాడటానికి సహాయపడతాయి.
అవును. ఒత్తిడి కార్టిసాల్ వంటి రసాయనాలను విడుదల చేస్తుంది, ఇవి అండం విడుదలను (Ovulation) అడ్డుకుంటాయి మరియు అండాల ఉత్పత్తిని తగ్గిస్తాయి. దీర్ఘకాలిక ఒత్తిడి హార్మోన్ల పనితీరును దెబ్బతీస్తుంది.
సంతానోత్పత్తికి అనుకూలమైన ఆహారం, సప్లిమెంట్లు మరియు జీవనశైలి మార్పులతో 30 రోజుల్లోనే అండం నాణ్యతను మెరుగుపరచుకోవడం సాధ్యమవుతుంది. ఆరోగ్యకరమైన ఆహారం మరియు హార్మోన్ల సమతుల్యత విజయవంతమైన గర్భధారణ అవకాశాలను పెంచుతాయి.
Take it as soon as you remember. If it is close to your next scheduled dose, call your doctor. Do not double-dose.
You might be having a mild reaction to the oil (sesame/peanut). If it spreads or becomes hives, tell your doctor; they may switch you to a different oil base (like olive oil).
Only if your doctor says so. The thigh muscle is tighter and often more painful for oil injections than the buttock.
Yes, mild discomfort due to digestion or the baby pushing is very normal. However, severe or persistent pain is not normal.
Pain caused by gas or indigestion does not harm the baby. However, pain caused by conditions like preeclampsia or liver issues can be dangerous for both mother and baby if left untreated.
If the pain is sudden, sharp, located on the upper right side, or accompanied by headache, vision changes, or vomiting, it is serious.
Call immediately if you have pain that doesn’t improve after an hour of rest, or if you have any bleeding, fever, or signs of high blood pressure.
Drinking cold milk, eating yogurt (curd), drinking ginger tea, and walking after meals are safe and effective natural remedies.
It is extremely rare (less than 1%) and carries a high risk of ectopic pregnancy. Medical assistance is highly recommended.
Success rates are generally high, often between 40-60% per cycle for women under 35, because these patients usually do not have underlying infertility issues.
No. Tying the tubes does not affect the ovaries or egg quality. Egg quality is determined primarily by your age.
One full cycle of IVF takes about 4 to 6 weeks from the start of stimulation medication to the pregnancy test.
For most women, especially those over 35 or with heavily damaged tubes, IVF is faster, safer, and has higher success rates than reversal surgery.
Yes! Coconut water is excellent during pregnancy. It is rich in electrolytes (potassium and magnesium) and helps with acidity. Count it towards your daily 3-liter goal.
- Flavoured Water: Yes, adding lemon, mint, or cucumber slices to water is a great way to make it tasty without sugar.
- ORS: Oral Rehydration Solution is safe but should only be used if you have diarrhoea or severe dehydration. Do not use it as a daily drink due to its salt and sugar content unless prescribed.
Try sucking on ice chips or homemade fruit popsicles. Drink water at room temperature or warm water with a drop of ginger juice instead of ice-cold water.
Yes, liquid foods like dal soup, tomato soup, and watery fruits contribute about 20% of your daily fluid intake.
Yes. The embryos undergo strict screening. The donors are tested for infectious diseases (HIV, Hepatitis, etc.) and genetic history before the embryos are accepted for donation.
We strive to match the donor’s physical characteristics (skin, eyes, hair, height) with yours as closely as possible. While genetics plays a role, the environment in the womb (epigenetics) also influences development.
It is relatively quick. Once a match is found and your cycle is synchronized, the preparation of the uterus takes about 2-3 weeks, followed immediately by the transfer.
Yes. In India, strict laws ensure that the identity of the donors is kept confidential and is never revealed to the recipients or the child.
Success rates for embryo donation are typically high, often ranging between 50% and 60%, because the embryos usually come from younger donors with proven fertility.
No. Under Indian law, all donors must be anonymous, and their identity must be kept strictly confidential. Therefore, you cannot meet your dono
Yes. As the selected donor is anonymous, their identity is kept completely private. All legal agreements ensure that their information is never shared.
The main document is a Donation Agreement, signed before treatment. Our team guides you through all legal steps.
No, absolutely not. The process involves providing a semen sample through masturbation, which is painless and non-invasive.
Donors usually donate once or twice a week. It is recommended to have 2-3 days of abstinence between donations to ensure high sperm count.
No. The male body produces millions of new sperm every day. Donating sperm does not run out your supply or affect your ability to have your own children later.
Yes. In India, donor anonymity is mandatory. Your identity will never be revealed to the parents or the child.
Sperm can be stored in liquid nitrogen indefinitely. However, clinics usually store it until the donation limit for that donor is reached or for a specific number of years as per clinic policy.
No, the procedure itself is not painful because it is done under mild sedation (anesthesia). You will sleep through the 15-minute process. You may feel some mild cramping, similar to period pain, for a day after the retrieval.
The active medical part of the process takes about 2 to 3 weeks. This includes the 10-12 days of injections and the egg retrieval day.
Under the current ART (Regulation) Act, 2021 in India, a woman can donate her eggs only once in her lifetime. This regulation is in place to protect the donor’s health.
No. A woman is born with millions of eggs. In a natural cycle, only one matures while hundreds die off. Egg donation simply “rescues” those eggs that would have been lost naturally that month. It does not deplete your reserve or affect your ability to have children in the future.
Yes, anonymity is mandatory under Indian law. Your identity, name, and address will never be revealed to the recipient couple or the child, and you will not know their identity either.
Fertility preservation refers to the medical techniques used to protect and store reproductive tissues, such as eggs, sperm, or embryos, to enable individuals to have children in the future, even if they face health challenges or age-related fertility decline.
Fertility preservation is essential for cancer patients undergoing treatments that may affect fertility, individuals with specific health conditions that could impair reproductive function, and those who are choosing to delay parenthood for personal or professional reasons.
The most common methods include freezing eggs, sperm, or embryos, as well as preserving ovarian tissue for future use.
Male factor infertility refers to infertility that is primarily caused by factors related to the male partner. It can include problems with sperm production, sperm quality, or sperm transport. Male factor infertility can significantly impact a couple’s ability to conceive, and both partners need to undergo a comprehensive analysis to identify the underlying causes and develop an appropriate treatment regimen.
Various methods can help diagnose male infertility. Alongside a meticulous medical history and physical examination, a semen examination is a vital diagnostic tool. This analysis evaluates sperm count, motility, morphology, and other factors that may impact fertility. Hormonal tests may also assess testosterone levels and other hormone imbalances that could contribute to infertility.
There are several treatment options available for low sperm count. Depending on the underlying condition, lifestyle modifications such as maintaining a healthy weight, lowering alcohol consumption, quitting smoking, and stress management can be beneficial. Certain medications can stimulate sperm production, and assisted reproductive techniques such as IUI) or in vitro fertilisation (IVF) can bypass low sperm count issues.
Nil sperm count, also known as azoospermia, can be treated depending on the specific cause. Surgical interventions like testicular sperm extraction (TESE) or micro-dissection TESE can retrieve sperm directly from the testes. These sperm can then be used in procedures like intracytoplasmic sperm injection (ICSI) during IVF. In some cases, donor sperm may be an option.
Success rates for fertility preservation vary significantly based on the chosen method and individual health factors. It is crucial to discuss these rates with a fertility specialist to understand personal chances.
It is advisable to start the fertility preservation process as early as possible. Prompt consultation with a healthcare provider can help determine the best timing and approach tailored to individual needs.
A semen analysis is a crucial test for evaluating male fertility. It assesses various semen parameters, including sperm count, motility, morphology, and volume. This analysis provides valuable information about the wellness and quality of the sperm, helping to diagnose any potential issues that may affect fertility.
A semen analysis is typically conducted by collecting a semen sample through masturbation. The sample is then examined under a microscope to assess various parameters. The process involves evaluating the sperm count, motility (ability to move), morphology (shape and size), and other factors such as pH levels and liquefaction time. The analysis provides valuable insights into the man’s fertility potential.
Male infertility can be treated through various approaches, depending on the underlying cause. Maintaining a healthy weight, quitting smoking, and limiting alcohol intake can positively influence sperm count. Fertility experts may also prescribe medications to address hormonal imbalances or stimulate sperm production. They can perform various surgical interventions to correct anatomical abnormalities, retrieve sperm, or alleviate obstructions.
Having a low sperm count does not mean you are infertile. Fertility is a complex issue that involves multiple factors, including the quality and motility of sperm. While a low sperm count may reduce the chances of natural conception, assisted reproductive techniques like IVF or IUI can still be successful in achieving pregnancy.
For couples experiencing infertility due to low sperm count, various treatment options are available. Fertility professionals may prescribe medications like Clomiphene citrate or hormone therapy to improve sperm production. Assisted reproductive techniques such as IUI, where sperm is directly placed into the uterus, or IVF with ICSI, where a single viable sperm is injected directly into an egg, are effective options.
Genetic testing is a medical diagnostic procedure that examines a person’s genes, chromosomes, or proteins to identify any changes or mutations. It helps assess risks for genetic conditions or the likelihood of passing them to future generations, playing a vital role in reproductive medicine and fertility planning.
Preimplantation Genetic Testing (PGT) is performed on embryos created via IVF. It involves testing a few cells from the embryo to detect genetic abnormalities, allowing fertility specialists to select the healthiest embryos for transfer, increasing pregnancy success and reducing the risk of genetic diseases.
Genetic testing is recommended for couples with a family history of genetic disorders, recurrent miscarriages, failed IVF attempts, or advanced maternal age. It helps in identifying potential genetic risks and supports personalized fertility treatment planning.
PGT-A screens embryos for chromosomal abnormalities like aneuploidy. It helps identify embryos with the correct number of chromosomes, improving implantation chances and reducing miscarriage risks or birth defects.
PGT-M identifies specific gene mutations or single-gene disorders in embryos. Its recommended for couples who are carriers of inherited disorders like autosomal recessive or X-linked conditions. This testing helps select embryos free from those genetic diseases.
PGT-SR detects structural changes in chromosomes such as translocations or inversions. It identifies embryos with normal structure, reducing the risk of implantation failure, miscarriage, or genetic conditions.
PGT significantly lowers the risk of genetic conditions like Thalassemia or Down Syndrome by selecting the healthiest embryos. However, it does not guarantee a healthy baby, and regular prenatal care during pregnancy is still necessary.
PGT is generally very safe. The main risk is a less than 1% chance of embryo damage during biopsy. There’s also a small chance the test may yield no results or that no normal embryos are found.
PGT increases the success rate of pregnancy by selecting chromosomally normal embryos, enhancing implantation and lowering miscarriage risk. This helps reduce the number of IVF cycles needed for a healthy pregnancy.
Yes, PGT is considered very safe. A few cells are taken from the embryo’s outer layer (future placenta), not the part that becomes the baby. It’s been widely used for years without affecting child health.
Female infertility refers to the inability of a woman to conceive a child after one year of regular, unprotected intercourse. Various factors, including ovulation disorders, hormonal imbalances, structural abnormalities in the reproductive organs, and age-related decline in fertility, can cause it. Female infertility can be emotionally challenging, but there are a variety of treatment options available to help women conceive and achieve their dream of starting a family.
Age has a significant influence on women’s fertility. As women age, the quantity and quality of their ova or eggs decline, making it more difficult to conceive. This decline in fertility starts around the age of 35 and accelerates after the age of 40. Age-related decline in fertility is primarily due to a decrease in the number of ova and an increase in chromosomal abnormalities. Understanding the influence of age on fertility is crucial, particularly if you’re over 35 and have been attempting to conceive for six months without success. Seeking medical assistance is advisable in such circumstances.
In many cases, female infertility does not have obvious signs or symptoms. However, some common signs that may indicate a potential fertility issue include irregular menstrual cycles, painful periods, excessive hair growth, or changes in libido. It is important to remember that the absence of these signs does not necessarily mean that you are not experiencing fertility issues. If you have been actively trying to conceive for a year without success, consult with a fertility specialist for a comprehensive evaluation.
Certain lifestyle habits can contribute to female infertility. These include smoking, excessive alcohol consumption, drug use, poor nutrition, and excessive exercise. These habits can negatively impact hormonal balance, ovulation, and reproductive health. Adopting healthy lifestyle habits when trying to conceive is essential. These include maintaining a balanced diet, exercising moderately, avoiding excessive alcohol drinking and smoking, and managing stress effectively. Making these lifestyle changes can significantly improve fertility outcomes.
Ovulation disorder is a condition where a woman’s ovaries do not release eggs regularly or do not release eggs at all. It can lead to female infertility, as without the release of a mature egg, fertilisation and pregnancy cannot occur. Ovulation disorders can occur due to various causes, such as hormonal imbalances, polycystic ovary syndrome (PCOS), thyroid disorders, or premature ovarian failure. Therefore, consulting with a fertility specialist to diagnose and treat ovulation disorders effectively is essential.
Uterine abnormalities are structural abnormalities in the uterus that can interfere with implantation and pregnancy. These abnormalities can include uterine fibroids, polyps, or a septum in the uterus. Uterine abnormalities can affect the ability of the fertilised egg to get implanted in the uterus and can increase the risk of miscarriage. Diagnosis and treatment of uterine abnormalities are essential for successful conception and a healthy pregnancy.
Diabetes can contribute to female infertility, mainly if not correctly managed. Diabetes can affect fertility by causing hormonal imbalances, irregular menstrual cycles, and anovulation. It can also increase the chance of complications during pregnancy. Proper management of diabetes through regular monitoring, lifestyle modifications, and medications can help improve fertility outcomes in women with diabetes.
Asherman’s Syndrome, also known as intrauterine adhesions, is a condition characterised by the formation of scar tissue inside the uterus. This scar tissue can lead to adhesions or fibrous bands that can obstruct the uterine cavity and interfere with implantation and pregnancy. Asherman’s Syndrome can develop due to previous uterine surgeries, including dilation and curettage (D&C;) or infections. It is a potential cause of infertility in women and requires medical intervention to restore normal uterine function.
Blastocyst culture does not increase the risk of multiples (twins, triplets) compared to traditional Day 2 or Day 3 embryo transfer. Blastocyst transfer reduces the chance of multiple pregnancies by allowing embryologists to select one healthiest embryos for transfer. By transferring high-quality embryo, the chances of a single, successful pregnancy is maximised while minimising the risks associated with multiple pregnancies. Blastocyst culture, in combination with PGT, provides individuals and couples with a safer and more controlled approach to achieving their dream of parenthood.
Blastocyst transfer significantly impacts the number of embryos transferred during an IVF cycle. By allowing embryos to develop until the blastocyst stage, embryologists can accurately assess their quality and select the healthiest embryos for transfer. This process enables them to transfer fewer embryos while maintaining high success rates. The ability to transfer single embryo reduces the risk of multiple pregnancies, which is associated with increased complications for both the mother and the babies. Blastocyst transfer provides individuals and couples a more controlled and safer approach to achieving a successful pregnancy.
The success rates of blastocyst transfer vary and depend on various factors, including the quality of the embryos, the age of the woman, and the specific circumstances of the individual or couple. However, studies have shown that blastocyst transfer generally results in higher pregnancy rates than traditional Day 2 or Day 3 embryo transfer. By allowing embryos to develop until the blastocyst stage, embryologists can select the healthiest embryos for transfer, increasing the possibility of successful implantation and pregnancy. The success rates of blastocyst transfer continue to improve with advancements in reproductive technology and the use of PGT.
Blastocyst transfer offers several advantages over traditional embryo transfer. By allowing embryos to develop for a more extended period, fertility specialists can accurately assess the quality of the embryos and select the healthiest ones for transfer. It improves the likelihood of successful implantation and pregnancy. Blastocyst transfer also reduces the risk of multiple pregnancies, as embryologistss can transfer a smaller number of high-quality embryos. Furthermore, blastocyst transfer provides a more natural and physiologically appropriate timeframe for embryo development, increasing the chances of a successful outcome.
Blastocyst transfer differs from traditional Day 2 or Day 3 embryo transfer in terms of embryo development stage at the time of transfer. In traditional embryo transfer, embryos are transferred into the uterus on either Day 2 or Day 3 after fertilisation, when they are still in the early stages of development. In contrast, blastocyst transfer involves transferring embryos on Day 5 or 6, when they have reached the blastocyst stage of development. This extended culture period allows embryologists to select the healthiest embryos with a higher chance of successful implantation and pregnancy.
Blastocyst culture is used in IVF to improve the selection process of embryos for transfer. By allowing embryos to develop longer, embryologists can identify the embryos with the highest chances of successful implantation and pregnancy. Blastocyst culture provides a more accurate assessment of embryo quality, as only the embryos that have reached the blastocyst stage are selected for transfer. This technique increases the success rates of IVF treatments and reduces the chance of multiple pregnancies by transferring a smaller number of high-quality embryos.
IUI (Intrauterine Insemination): Specially prepared sperm are placed directly into the uterus around the time of ovulation. Fertilization happens inside the body.
IVF (In Vitro Fertilization): Eggs are retrieved from the ovaries and fertilized by sperm outside the body, in a lab. The resulting embryo is then transferred into the uterus.
Blastocyst culture is a laboratory method used during IVF treatments to allow embryos to develop for a more extended period before transfer. We perform blastocyst transfer to increase the chances of implantation. Additionally, if the embryo can reach the blastocyst level in the lab, it is considered to be genetically viable, which improves the pregnancy rate and allows for single embryo transfer.
Common medications used in IVF include:
– Ovarian Stimulation Drugs: Help the ovaries produce multiple eggs (e.g., FSH, hMG).
– Ovulation Prevention Drugs: Prevent premature ovulation (e.g., GnRH agonists or antagonists).
– Trigger Shot: Stimulates final egg maturation before retrieval (e.g., hCG).
– Progesterone: Prepares the uterine lining for embryo implantation and supports early pregnancy.
The number of injections depends on your treatment plan. Typically, 1 to 2 injections per day are required for about 8–12 days during the ovarian stimulation phase. Progesterone support may continue for several weeks through injections or alternative methods like pessaries or gels.
Yes, PICSI significantly enhances embryo quality. This is evidenced by higher implantation potential and a reduced risk of aneuploidies, which are abnormalities in chromosome numbers.
– Injections: May cause mild stinging or bruising at the site.
– Egg Retrieval: Done under sedation or light anesthesia, typically pain-free. Some cramping may occur afterward.
– Embryo Transfer: A quick and usually painless procedure, similar to a Pap smear. Some women may feel mild cramping.
Research comparing PICSI with standard ICSI using sibling oocytes has shown that PICSI achieves significantly higher fertilisation rates, improves rates of embryos suitable for transfer, and produces a greater number of high-quality embryos.
IVF success rates vary based on age, cause of infertility, embryo quality, and clinic expertise. Women under 35 generally have a 60–70% success rate per cycle. Success rates decline with age. Your fertility specialist can provide individual success estimates.
PICSI operates by exposing sperm to hyaluronic acid (HA), a naturally occurring compound in the human body. The process identifies sperm that can bind to HA, and these are then selected for use in fertility treatments.
The number of IVF cycles needed varies per person. Some achieve pregnancy in the first cycle, while others may require multiple cycles. Your doctor will evaluate your response and outcomes from the first cycle to plan further treatment.
The primary benefit of PICSI is its advanced method for selecting the best sperm. This technique enables embryologists to differentiate between mature and immature sperm. Mature sperm have fully developed and are less likely to contain damaged DNA or an incorrect number of chromosomes, making them preferable for use in treatments.
– Physically: You will likely get your period, which may be heavier than usual. Hormonal levels will gradually return to normal.
– Emotionally: A failed IVF cycle can be emotionally tough. Feelings of sadness, anger, or grief are common. Emotional support or counselling is recommended.
The overall risk of birth defects in IVF babies is low and comparable to naturally conceived babies. Some studies suggest a slight increase in specific conditions, possibly linked to the cause of infertility rather than IVF itself. Preimplantation Genetic Testing (PGT) can help screen embryos.
An IVF pregnancy is generally considered safer after a fetal heartbeat is confirmed via ultrasound, typically around 6–7 weeks. After the first trimester (around 12 weeks), IVF pregnancies are managed similarly to natural pregnancies, though your doctor may recommend additional monitoring.
The main difference is how fertilization occurs. In IVF, sperm and eggs are mixed for natural fertilization. In ICSI, a single sperm is directly injected into the egg by an embryologist.
No, IUI is generally not painful. Some women may experience mild, temporary cramping during or after the procedure. The entire process is quick and usually completed within a few minutes.
ICSI is generally safe. Possible risks include ovarian hyperstimulation syndrome (OHSS), multiple pregnancies if more than one embryo is transferred, and very rare chances of egg damage during injection, especially minimized when performed by skilled embryologists.
IUI stands for Intrauterine Insemination. It is a fertility procedure in which specially prepared sperm is directly inserted into a woman’s uterus to increase the chances of fertilization.
No, ICSI is painless as its performed in the lab. Patients may experience mild discomfort from hormone injections and manageable cramps after egg retrieval, which is done under anesthesia.
The success rate of IUI treatment ranges from 15% to 20% per cycle. Success depends on several factors, including the woman’s age, cause of infertility, and sperm quality. Most successful pregnancies occur within the first 3 to 4 IUI cycles.
ICSI failure can be due to poor egg quality (often age-related), poor embryo development from genetic issues, or implantation failure caused by uterine or endometrial conditions. These are biological factors addressed through detailed diagnostics.
The cost of IUI treatment in India is relatively affordable and typically ranges from ₹5,000 to ₹15,000 per cycle. Prices may vary depending on the clinic, city, and whether additional medications or scans are required.
The main difference is where fertilization occurs. In IUI, sperm is inserted into the uterus, and fertilization happens naturally inside the body. In IVF, eggs are retrieved, fertilized with sperm in a lab, and the resulting embryo is transferred into the uterus.
IUI and IVF serve different purposes. IUI is a simpler, less invasive, and more affordable treatment often recommended for mild infertility cases. IVF is more advanced and typically used when IUI fails or for complex fertility issues. The best option depends on individual circumstances.
Most fertility specialists recommend trying 3 to 4 IUI cycles. If pregnancy is not achieved after that, IVF is often suggested due to its higher success rates in complex or persistent infertility cases.
The IUI procedure itself takes only a few minutes. However, the full IUI cycle — including ovulation monitoring, medication (if needed), and timing the procedure — aligns with the menstrual cycle and typically takes about 2 to 3 weeks.
ఆరోగ్యకరమైన జీవనశైలిని పాటించడం ద్వారా దీనిని నివారించవచ్చు. సమతుల్య ఆహారం (Balanced diet) తీసుకోవడం, రోజూ వ్యాయామం చేయడం, ఒత్తిడిని తగ్గించుకోవడం మరియు క్రమం తప్పకుండా హెల్త్ చెకప్లు చేయించుకోవడం ద్వారా హార్మోన్ల ఆరోగ్యాన్ని కాపాడుకోవచ్చు.
మంచి ఆహారం, కంటి నిండా నిద్ర, యోగా లేదా ధ్యానం మరియు ధూమపానం/మద్యపానానికి దూరంగా ఉండటం వల్ల హార్మోన్ల స్థాయిలు అదుపులో ఉంటాయి. ఇవి సహజంగానే హార్మోన్ల సమతుల్యతను మెరుగుపరుస్తాయి.
శరీరంలో హార్మోన్ల స్థాయిలు మరీ ఎక్కువగా లేదా మరీ తక్కువగా ఉండటాన్ని హార్మోన్ల అసమతుల్యత (Hormonal imbalance) అంటారు. దీనివల్ల సంతానలేమి (Infertility), నెలసరి సరిగా రాకపోవడం, బరువు పెరగడం మరియు విపరీతమైన అలసట వంటి సమస్యలు వస్తాయి.
హార్మోన్లు అండం విడుదల (Ovulation), గర్భాశయ ఆరోగ్యం మరియు పురుషులలో వీర్య కణాల ఉత్పత్తిని (Sperm production) నియంత్రిస్తాయి. ఈస్ట్రోజెన్, ప్రొజెస్టెరాన్ మరియు టెస్టోస్టెరాన్ వంటి హార్మోన్లు సరైన మోతాదులో ఉంటేనే గర్భం దాల్చడం సాధ్యమవుతుంది.
అవును. కెఫిన్ (కాఫీ/టీ) ఎక్కువగా తీసుకుంటే సంతానోత్పత్తి తగ్గడం, గర్భస్రావం అయ్యే ప్రమాదం మరియు అండం నాణ్యత దెబ్బతినే అవకాశం ఉంది. పునరుత్పత్తి ఆరోగ్యం కోసం కెఫిన్ను తగ్గించడం మంచిది.
హార్మోన్ల సమతుల్యతకు నిద్ర చాలా ముఖ్యం. సరిగ్గా నిద్రపోకపోతే హార్మోన్ల ఉత్పత్తి దెబ్బతిని, అండం విడుదల (Ovulation) మరియు నెలసరి చక్రాలపై చెడు ప్రభావం పడుతుంది.
మద్యం హార్మోన్ల అసమతుల్యతకు కారణమవుతుంది. మహిళల్లో నెలసరిని దెబ్బతీస్తుంది. పురుషులలో వీర్య కణాల నాణ్యత మరియు సంఖ్యను తగ్గిస్తుంది, దీనివల్ల గర్భం రావడం కష్టమవుతుంది.
అవును, ధూమపానం హార్మోన్ల స్థాయిలను దెబ్బతీస్తుంది మరియు పునరుత్పత్తి అవయవాలకు రక్త ప్రసరణను తగ్గిస్తుంది. ఇది అండం అభివృద్ధిని అడ్డుకుని, ఫలదీకరణ అవకాశాలను తగ్గిస్తుంది.
అధిక బరువు హార్మోన్ల స్థాయిలను దెబ్బతీసి, ఇన్సులిన్ నిరోధకతను పెంచుతుంది. దీనివల్ల అండం విడుదల సరిగా జరగదు మరియు అండం నాణ్యత తగ్గి, గర్భం దాల్చే అవకాశాలు సన్నగిల్లుతాయి.
గర్భం దాల్చడానికి ఉత్తమ వయస్సు 20లు లేదా 30ల ప్రారంభం. ఈ సమయంలో అండాల సంఖ్య మరియు నాణ్యత చాలా బాగుంటాయి. 35 ఏళ్ల తర్వాత సంతానోత్పత్తి సామర్థ్యం, అండం నాణ్యత క్రమంగా తగ్గుతాయి.
బ్లాక్ అయిన ట్యూబ్స్ నేరుగా గర్భస్రావానికి కారణం కావు, కానీ అవి ఎక్టోపిక్ ప్రెగ్నెన్సీ ప్రమాదాన్ని పెంచుతాయి. అండం మరియు వీర్య కణం కలవకుండా అడ్డుకోవడం వల్ల ఇవి ప్రధానంగా సంతానలేమికి (Infertility) దారితీస్తాయి.
HSG లేదా లాపరోస్కోపీ ద్వారా సమస్యను నిర్ధారిస్తారు. చికిత్సగా చిన్నపాటి సర్జరీ లేదా IVF ను సూచించవచ్చు. రికవరీ సమయం చికిత్స పద్ధతిపై ఆధారపడి ఉంటుంది.
ఇన్ఫెక్షన్లు లేదా ఎండోమెట్రియోసిస్ వల్ల ట్యూబ్స్ మూసుకుపోవడాన్ని ఇది సూచిస్తుంది. దీనివల్ల అండం వీర్య కణంతో కలవలేదు. సహజ ఫలదీకరణ ప్రక్రియకు ఆటంకం కలిగించి, మహిళ గర్భం దాల్చడాన్ని కష్టతరం చేస్తుంది.
సిద్ధం కావడంలో సమగ్ర వైద్య పరీక్షలు, చికిత్సకు ముందు డాక్టర్ ఇచ్చిన సూచనలను పాటించడం, సాధారణ ఆరోగ్యాన్ని మెరుగుపరచుకోవడం మరియు ప్రక్రియ అంతటా వైద్య బృందంతో ఎప్పటికప్పుడు మాట్లాడటం ఉంటాయి.
పిండ బదిలీకి ముందు మరియు తర్వాత చికిత్సా విధానాలు జీవనశైలి మార్పుల నుండి అధునాతన వైద్య చికిత్సల వరకు ఉంటాయి. సరైన వ్యూహం అనేది వ్యక్తిగత సమస్య, తీవ్రత మరియు రోగి ప్రాధాన్యతలపై ఆధారపడి ఉంటుంది.
సంతానోత్పత్తి సవాళ్లు, అసాధారణ లక్షణాలు లేదా పునరుత్పత్తి ఆరోగ్యం గురించి ఆందోళనలు ఉన్నప్పుడు సంప్రదించాలి. ఫెర్టిలిటీ నిపుణులతో ముందస్తు సంప్రదింపులు సమస్యలను గుర్తించడానికి మరియు సకాలంలో చికిత్సలు తీసుకోవడానికి సహాయపడతాయి.
దీని ప్రభావం వయస్సు, మొత్తం ఆరోగ్యం మరియు నిర్దిష్ట వైద్య చరిత్రతో సహా వ్యక్తిగత కారకాలపై మారుతుంది. చికిత్సా పద్ధతులను ఆప్టిమైజ్ చేయడానికి మరియు విజయ శాతాలను గరిష్టంగా పెంచడానికి ఫెర్టిలిటీ నిపుణులు ప్రతి కేసును అంచనా వేస్తారు.
సమాచారంతో కూడిన సంతానోత్పత్తి నిర్ణయాలు తీసుకోవడానికి ఈ విషయాలను అర్థం చేసుకోవడం ముఖ్యం. వ్యక్తిగత పరిస్థితుల ఆధారంగా తగిన చికిత్సా విధానాలను మరియు ఆశించిన ఫలితాలను నిర్ణయించడానికి ఫెర్టిలిటీ నిపుణుల సమగ్ర మూల్యాంకనం అవసరం.
Yes. While most calculators assume a 28-day cycle, an accurate period calculator for irregular cycles requires 6 months of data to find your specific average and deviation. Learn how to track irregular cycles here.
It uses your “Last Period Date” and “Average Cycle Length” to project future dates. Our tool acts as a Menstrual Phase Calculator, identifying exactly when your next cycle begins.
Accuracy depends on consistent data entry. By tracking the first day of bleeding for 3+ months, our calculator becomes significantly more precise for your body.
Yes, the implantation window is typically between Day 6 and Day 10, so it is very possible for implantation to occur on Day 8.
Mild cramps, light spotting, breast tenderness, and fatigue are all possible signs. However, the absence of these symptoms is also completely normal in a successful pregnancy.
No, not at all. Many women with successful IVF pregnancies report having no early symptoms.
You should wait for the official beta hCG blood test scheduled by your doctor, usually 10-14 days after the transfer, for the most accurate result.
The progesterone medication you take is designed to support the pregnancy and can cause symptoms that are identical to early pregnancy signs, such as bloating and breast soreness.
Yes, mild, dull cramping or a light pulling sensation is very normal and can be a sign of implantation or your uterus adjusting.
It is not recommended. A home test on Day 10 may not be accurate and can lead to a false negative. It’s best to wait for the official blood test scheduled by your doctor.
Light brown or pink spotting around this time can be a positive sign of implantation. However, if the bleeding is bright red or heavy, you should contact your doctor.
This is completely normal. Many women have no symptoms at all and go on to have a healthy pregnancy. A lack of symptoms is not a sign that the transfer has failed.
Stay busy with relaxing activities you enjoy. Practice deep breathing or meditation, connect with your partner, and try to avoid spending too much time searching for symptoms online. Trust the process and your medical team.
ఇది ప్లేట్లెట్స్ (రక్తం గడ్డకట్టే కణాలు) తగ్గే సమస్య. గర్భధారణ సమయంలో తల్లి రక్తం ద్వారా బిడ్డకు కూడా దీని ప్రభావం పడే అవకాశం ఉంది కాబట్టి, నిరంతర వైద్య పర్యవేక్షణ అవసరం.
Cramping that occurs 6–12 days after IUI could be implantation cramping — a sign that a fertilised egg has attached to the uterine wall. Implantation cramps typically feel like a mild, short-lived pinching sensation and may come with light spotting. However, cramping alone is not a reliable pregnancy indicator, as it can also be caused by the procedure itself or ovulation. The only way to confirm pregnancy is through a beta hCG blood test, which doctors recommend at 14 days post-IUI.
Positive signs of implantation include light spotting (pink or brown), mild cramping or “tugging” sensations, and breast tenderness. However, since these often mimic PMS or the side effects of fertility medications, the only definitive confirmation is a blood test about two weeks post-procedure.
Yes, implantation must happen first. It is the physical attachment of the embryo that signals your body to start producing hCG, which then takes a few days to build up to a level high enough for a pregnancy test to detect.
ఫెర్టిలిటీ ప్రిజర్వేషన్ అనేది ఒక వైద్య ప్రక్రియ. దీని ద్వారా అండాలు (eggs), శుక్రకణాలు (sperm), లేదా పిండాలను (embryos) వంటి పునరుత్పత్తి కణజాలాలను భద్రపరుస్తారు. భవిష్యత్తులో అనారోగ్య సమస్యలు లేదా వయసు పెరగడం వల్ల సంతాన సామర్థ్యం తగ్గినా, పిల్లలను కనడానికి వీలు కల్పించడమే దీని ముఖ్య ఉద్దేశ్యం.
ఫెర్టిలిటీ ప్రిజర్వేషన్ ముఖ్యంగా ఈ క్రింది వారికి అవసరం:
- సంతాన సామర్థ్యంపై ప్రభావం చూపే చికిత్సలు (ఉదా: క్యాన్సర్ చికిత్స) తీసుకుంటున్న వారికి.
- సంతానోత్పత్తిని దెబ్బతీసే ఇతర ఆరోగ్య సమస్యలు ఉన్నవారికి.
- వ్యక్తిగత లేదా వృత్తిపరమైన కారణాల వల్ల పిల్లలను కనడాన్ని వాయిదా వేయాలనుకునే వారికి.
అండాలు (eggs), శుక్రకణాలు (sperm), లేదా పిండాలను (embryos) గడ్డకట్టించడం మరియు భవిష్యత్ ఉపయోగం కోసం అండాశయ కణజాలాన్ని (ovarian tissue) భద్రపరచడం వంటివి అత్యంత సాధారణ పద్ధతులు.
సక్సెస్ రేట్లు అనేవి ఎంచుకున్న పద్ధతి, వ్యక్తి యొక్క ఆరోగ్యం మరియు వయసు వంటి అనేక అంశాలపై ఆధారపడి ఉంటాయి. కాబట్టి ఇవి ఒకరి నుండి మరొకరికి మారుతూ ఉంటాయి. మీ వ్యక్తిగత విజయావకాశాలను తెలుసుకోవడానికి ఫెర్టిలిటీ నిపుణులతో ఈ రేట్ల గురించి చర్చించడం చాలా ముఖ్యం.
ఫెర్టిలిటీ ప్రిజర్వేషన్ ప్రక్రియను వీలైనంత త్వరగా ప్రారంభించడం మంచిది. వెంటనే వైద్యుడిని లేదా ఫెర్టిలిటీ నిపుణుడిని సంప్రదించడం ద్వారా, మీ వ్యక్తిగత అవసరాలకు తగిన సరైన సమయం మరియు పద్ధతిని వారు నిర్ధారించగలరు.
ఫెర్టిలిటీ ప్రిజర్వేషన్ అనేది, భవిష్యత్తులో ఉపయోగించుకోవడానికి వీలుగా ఒక వ్యక్తి తమ సంతాన సామర్థ్యాన్ని కాపాడుకోవడానికి వీలు కల్పించే ఒక వైద్య ప్రక్రియ. క్యాన్సర్ చికిత్సలు, వయసు పైబడటం, లేదా కొన్ని రకాల ఆరోగ్య సమస్యల వంటి కారణాల వల్ల తమ సంతాన సామర్థ్యం దెబ్బతినే అవకాశం ఉన్నవారికి ఇది ప్రత్యేకంగా ప్రయోజనకరంగా ఉంటుంది. భవిష్యత్తులో సొంత బిడ్డలను కనాలనుకునే వారికి, వారి వీర్యకణాలు, అండాలు, లేదా పిండాలను భద్రపరచడం ద్వారా ఫెర్టిలిటీ ప్రిజర్వేషన్ ఒక కొత్త ఆశను అందిస్తుంది.
క్యాన్సర్ చికిత్సలు, సర్జరీలు, లేదా కొన్ని రకాల ఆరోగ్య సమస్యల వంటి వివిధ కారణాల వల్ల తమ సంతాన సామర్థ్యాన్ని కోల్పోయే ప్రమాదం ఉన్నవారికి ఫెర్టిలిటీ ప్రిజర్వేషన్ అందిస్తారు. ఈ చికిత్సలు మరియు ఆరోగ్య సమస్యలు ప్రత్యుత్పత్తి సామర్థ్యాలను తీవ్రంగా దెబ్బతీసి, సహజంగా గర్భం దాల్చడాన్ని కష్టతరం లేదా అసాధ్యం చేస్తాయి. ఫెర్టిలిటీ ప్రిజర్వేషన్, వ్యక్తులు ఈ చికిత్సలను ప్రారంభించడానికి ముందే తమ వీర్యకణాలు లేదా అండాలు, పిండాలను భద్రపరచుకునే అవకాశాన్ని ఇస్తుంది. ఇది భవిష్యత్తులో విజయవంతంగా గర్భం దాల్చి, సొంత బిడ్డలను కనే అవకాశాలను పెంచుతుంది.
తమ సంతాన సామర్థ్యాన్ని కోల్పోయే ప్రమాదం ఎక్కువగా ఉన్న వ్యక్తులు ఫెర్టిలిటీ ప్రిజర్వేషన్ను పరిగణించవచ్చు. ఉదాహరణకు, కీమోథెరపీ లేదా రేడియేషన్ థెరపీ వంటి క్యాన్సర్ చికిత్సలు తీసుకోబోయే వారు. వ్యక్తిగత లేదా వృత్తిపరమైన కారణాల వల్ల కుటుంబాన్ని ఆలస్యంగా ప్రారంభించాలని ప్లాన్ చేసుకునే వారికి కూడా ఇది ప్రయోజనకరంగా ఉంటుంది. అంతేకాకుండా, కొన్ని రకాల ఆరోగ్య సమస్యలు ఉన్నవారు లేదా లింగ మార్పిడి చేయించుకుంటున్న వారు కూడా భవిష్యత్తు కోసం తమ ప్రత్యుత్పత్తి ఎంపికలను కాపాడుకోవడానికి ఫెర్టిలిటీ ప్రిజర్వేషన్ను పరిగణించవచ్చు.
సోషల్ ఫ్రీజింగ్ అనేది, భవిష్యత్తులో ఉపయోగించుకోవడానికి వీలుగా ఒక వ్యక్తి తమ అండాలను భద్రపరచుకునే ఒక ఫెర్టిలిటీ ప్రిజర్వేషన్ ఎంపిక. దీనిని ‘ఎలక్టివ్ ఎగ్ ఫ్రీజింగ్’ అని కూడా అంటారు. ముఖ్యంగా వ్యక్తిగత లేదా వృత్తిపరమైన కారణాల వల్ల పిల్లలను కనడాన్ని వాయిదా వేయాలనుకునే మహిళలకు ఇది ప్రయోజనం చేకూరుస్తుంది. సోషల్ ఫ్రీజింగ్, మహిళలు తక్కువ వయసులోనే తమ అండాలను భద్రపరచుకునే అవకాశాన్ని ఇస్తుంది. తక్కువ వయసులో అండాలు అధిక నాణ్యతతో ఉంటాయి కాబట్టి, భవిష్యత్తులో వాటిని ఉపయోగించినప్పుడు విజయవంతంగా గర్భం దాల్చే అవకాశం ఎక్కువగా ఉంటుంది.
ఎగ్ ఫ్రీజింగ్, లేదా ఊసైట్ క్రయోప్రిజర్వేషన్, అనేది భవిష్యత్తులో ఉపయోగించుకోవడానికి వీలుగా ఒక మహిళ యొక్క అండాలను సేకరించి, నిల్వ చేసే ప్రక్రియ. ఈ ప్రక్రియ, అండాశయాలలో బహుళ అండాలు అభివృద్ధి చెందడాన్ని ప్రోత్సహించడానికి హార్మోన్ల ఉత్తేజంతో మొదలవుతుంది. అండాలు సరైన పరిమాణానికి పెరిగాక, అల్ట్రాసౌండ్ పర్యవేక్షణలో, శరీరానికి తక్కువ శ్రమ కలిగించే ట్రాన్స్వజైనల్ పద్ధతి ద్వారా వాటిని సేకరిస్తారు. ఇలా సేకరించిన అండాలను, వాటి జీవశక్తిని కాపాడటానికి విట్రిఫికేషన్ అనే టెక్నిక్తో అత్యంత వేగంగా గడ్డకట్టిస్తారు. ఈ భద్రపరిచిన అండాలను ఫెర్టిలిటీ కేంద్రాలు ఎక్కువ కాలం పాటు నిల్వ చేయగలవు మరియు మహిళ వాటిని సంతాన చికిత్సల కోసం ఉపయోగించడానికి సిద్ధంగా ఉన్నప్పుడు, వాటిని సాధారణ స్థితికి తీసుకువచ్చి ఉపయోగిస్తారు.
ఎగ్ ఫ్రీజింగ్ విజయం అనేక అంశాలపై ఆధారపడి ఉంటుంది. వీటిలో, అండాలను భద్రపరిచే సమయంలో మహిళ వయసు, అండాల నాణ్యత, మరియు వాటిని గడ్డకట్టించడానికి, తిరిగి సాధారణ స్థితికి తీసుకురావడానికి ఉపయోగించే పద్ధతి ముఖ్యమైనవి. తక్కువ వయసున్న మహిళలకు సాధారణంగా మంచి నాణ్యత గల అండాలు ఎక్కువగా ఉంటాయి కాబట్టి, వారికి విజయావకాశాలు ఎక్కువగా ఉంటాయి. Ferty9 ఫెర్టిలిటీ సెంటర్లో ఉపయోగించే విట్రిఫికేషన్ టెక్నిక్, మంచు స్ఫటికాలు ఏర్పడటాన్ని నివారించి, అండాల నాణ్యతను కాపాడటం ద్వారా ఎగ్ ఫ్రీజింగ్ సక్సెస్ రేట్లను గణనీయంగా మెరుగుపరిచింది. అంతేకాకుండా, ఈ ప్రక్రియను చేసే ఫెర్టిలిటీ నిపుణుల నైపుణ్యం మరియు అనుభవం కూడా ఎగ్ ఫ్రీజింగ్ విజయంలో కీలక పాత్ర పోషిస్తాయి. మా Ferty9 ఫెర్టిలిటీ సెంటర్లోని బృందంలో, అత్యంత నైపుణ్యం కలిగిన నిపుణులు ఉన్నారు. వీరు భద్రపరిచిన అండాలను ఉపయోగించి గర్భం దాల్చే అవకాశాలను గరిష్టంగా పెంచడానికి మరియు ఉత్తమమైన సంరక్షణను అందించడానికి అంకితభావంతో పనిచేస్తారు.
ఎగ్ ఫ్రీజింగ్ అనేది చాలా సురక్షితమైన మరియు శరీరం బాగా తట్టుకోగల ప్రక్రియ. దీనివల్ల సమస్యలు వచ్చే ప్రమాదం చాలా తక్కువ. అయినప్పటికీ, ఏ వైద్య ప్రక్రియలోనైనా ఉన్నట్లుగానే, ఇందులో కూడా కొన్ని ఇబ్బందులు సంభవించవచ్చు. అండాశయాలను ఉత్తేజపరచడానికి ఉపయోగించే హార్మోన్ ఇంజెక్షన్ల వల్ల కలిగే సాధారణ దుష్ప్రభావాలలో కడుపులో తేలికపాటి ఉబ్బరం, రొమ్ములలో సున్నితత్వం (నొప్పిగా అనిపించడం), మరియు మూడ్ స్వింగ్స్ (మానసిక స్థితిలో ఆకస్మిక మార్పులు) ఉంటాయి. ఈ దుష్ప్రభావాలు తాత్కాలికమైనవి మరియు చికిత్స పూర్తయిన తర్వాత సాధారణంగా వాటంతట అవే తగ్గిపోతాయి. అరుదైన సందర్భాల్లో, ఒవేరియన్ హైపర్స్టిమ్యులేషన్ సిండ్రోమ్ (OHSS) వచ్చే ప్రమాదం ఉంది. ఇది పొత్తికడుపు మరియు ఛాతీలో అధికంగా ద్రవం చేరే పరిస్థితి. అయినప్పటికీ, జాగ్రత్తగా పర్యవేక్షించడం మరియు ప్రతి వ్యక్తికి ప్రత్యేకంగా రూపొందించిన చికిత్సా పద్ధతులను పాటించడం ద్వారా OHSS ప్రమాదాన్ని చాలా వరకు తగ్గించవచ్చు. మా Ferty9 ఫెర్టిలిటీ సెంటర్లో, మా అనుభవజ్ఞులైన నిపుణులు ప్రతి రోగిని ఎగ్ ఫ్రీజింగ్ ప్రక్రియ అంతటా నిశితంగా పర్యవేక్షిస్తూ, వారి భద్రత మరియు శ్రేయస్సును నిర్ధారిస్తారు.
స్పెర్మ్ ఫ్రీజింగ్, లేదా స్పెర్మ్ క్రయోప్రిజర్వేషన్, అనేది ఒక వీర్య నమూనాను సేకరించి, భవిష్యత్తులో ఉపయోగించుకోవడానికి వీలుగా దానిని గడ్డకట్టించి భద్రపరిచే ప్రక్రియ. వీర్యాన్ని భద్రపరచడం ద్వారా, వ్యక్తులు తమ సంతాన సామర్థ్యాన్ని కాపాడుకోవచ్చు మరియు ఇన్ విట్రో ఫెర్టిలైజేషన్ (IVF) లేదా ఇంట్రాయూటరైన్ ఇన్సెమినేషన్ (IUI) వంటి సహాయక ప్రత్యుత్పత్తి పద్ధతుల కోసం తమ వీర్యాన్ని ఉపయోగించుకునే అవకాశం ఉంటుంది.
ఎంబ్రియో ఫ్రీజింగ్, లేదా ఎంబ్రియో క్రయోప్రిజర్వేషన్, అనేది సహాయక ప్రత్యుత్పత్తి సాంకేతికతలో ఉపయోగించే ఒక పద్ధతి. ఇందులో, భవిష్యత్తులో ఉపయోగించుకోవడానికి వీలుగా పిండాలను గడ్డకట్టించి, నిల్వ చేయడం జరుగుతుంది.
పిండాలను గడ్డకట్టించడం ద్వారా చాలా సంవత్సరాల పాటు సురక్షితంగా నిల్వ చేయవచ్చు. పిండాలను ఎంతకాలం నిల్వ చేయవచ్చు అనేది ఫెర్టిలిటీ కేంద్రం యొక్క నిబంధనలు మరియు వ్యక్తులు లేదా జంటల ఇష్టాయిష్టాలు వంటి వివిధ అంశాలపై ఆధారపడి ఉంటుంది.
చర్మంపై కమిలిన మచ్చలు వచ్చినా, చిగుళ్ళ నుండి రక్తం వచ్చినా లేదా పీరియడ్స్లో అధిక రక్తస్రావం అయినా వెంటనే డాక్టర్ని కలవాలి. చికిత్స సమయంలో రెగ్యులర్ చెకప్స్ తప్పనిసరి.
ప్రధానంగా రక్తస్రావం ఎక్కువయ్యే ప్రమాదం ఉంటుంది. డెలివరీ సమయంలో ఇబ్బందులు రావచ్చు మరియు పుట్టబోయే బిడ్డలో కూడా ప్లేట్లెట్స్ తగ్గే అవకాశం ఉంటుంది.
ప్లేట్లెట్స్ తగ్గకుండా మందులు వాడటం, డాక్టర్ల సూచనలు పాటించడం, రెగ్యులర్ బ్లడ్ టెస్టులు మరియు మంచి ఆహారం తీసుకోవడం ద్వారా సక్సెస్ రేట్ పెంచుకోవచ్చు.
కచ్చితంగా చేయించుకోవచ్చు. నిపుణుల పర్యవేక్షణలో ప్లేట్లెట్స్ అదుపులో ఉంచుకుంటే, సాధారణ మహిళల్లాగే మీరు కూడా తల్లిదండ్రులు కావచ్చు.
సాధారణంగా, IVF పద్ధతిలో ఒకటి లేదా రెండు బ్లాస్టోసిస్ట్లను (ఎంబ్రియోలను) గర్భంలోకి ప్రవేశపెడతారు. గర్భం దాల్చే అవకాశాలను పెంచడానికి, అదే సమయంలో కవలలు లేదా ముగ్గురు పిల్లలు పుట్టే రిస్క్ (Multiple Pregnancies) తగ్గించడానికి డాక్టర్లు ఈ నిర్ణయం తీసుకుంటారు. ఒకవేళ పేషెంట్ వద్ద మంచి నాణ్యమైన ఎంబ్రియోలు (High-quality embryos) ఉంటే, ఆరోగ్య సమస్యలు రాకుండా ఉండటానికి కేవలం ఒక్క ఎంబ్రియోని మాత్రమే ట్రాన్స్ఫర్ చేయమని (Single Embryo Transfer) డాక్టర్లు సలహా ఇస్తారు.
అవును, డే 3 ట్రాన్స్ఫర్ కంటే డే 5 బ్లాస్టోసిస్ట్ ట్రాన్స్ఫర్ చాలా మంచిది. దీనికి కొన్ని ముఖ్యమైన కారణాలు ఉన్నాయి:
- ఎదుగుదల: 5వ రోజుకు ఎంబ్రియో మరింత బాగా అభివృద్ధి చెందుతుంది.
- అతుక్కునే సామర్థ్యం: ఈ దశలో ఉన్న ఎంబ్రియో గర్భసంచికి అతుక్కునే అవకాశం (Implantation potential) చాలా ఎక్కువ.
- సింక్రనైజేషన్: సహజంగా ఎంబ్రియో గర్భసంచిలోకి చేరే సమయానికి (5వ రోజు), గర్భసంచి పొర కూడా సిద్ధంగా ఉంటుంది. దీనివల్ల పొరపాట్లు జరిగే అవకాశం తగ్గుతుంది.
- అలాగే, ఎంబ్రియో నాణ్యతను ఖచ్చితంగా తెలుసుకోవడానికి మరియు తక్కువ ఎంబ్రియో ట్రాన్స్ఫర్ చేయడం ద్వారా కవలలు పుట్టే రిస్క్ తగ్గించడానికి ఇది సహాయపడుతుంది.
IVF విజయం సాధించడంలో ఎంబ్రియో (పిండం) నాణ్యత చాలా కీలకమైన పాత్ర పోషిస్తుంది. ఎంబ్రియో ఎంత ఆరోగ్యంగా ఉంటే (High-quality), అది గర్భసంచికి అతుక్కుని, ఆరోగ్యకరమైన బిడ్డగా పెరిగే అవకాశం అంత ఎక్కువగా ఉంటుంది. ఎంబ్రియో ఏ దశలో ఉంది? దాని ఆకారం (Morphology) ఎలా ఉంది? మరియు దాని జన్యుపరమైన ఆరోగ్యం (Genetic health) ఎలా ఉంది? అనే అంశాలు IVF సక్సెస్ రేట్ను నిర్ణయిస్తాయి. అందుకే నాణ్యమైన ఎంబ్రియో (ఎంబ్రియోని) ఎంచుకోవడం చాలా ముఖ్యం.
An endocrine disorder is a medical condition where your body’s glands produce too much or too little of a specific hormone, disrupting your body’s natural chemical balance.
Not directly. High cholesterol is a metabolic issue. However, it is often caused by endocrine disorders like hypothyroidism (underactive thyroid) or uncontrolled diabetes.
Yes. Diabetes is one of the most common endocrine disorders. It involves the pancreas (an endocrine gland) failing to produce enough insulin or the body failing to use it effectively.
Yes. Polycystic Ovary Syndrome (PCOS) is the most common endocrine condition in women of reproductive age. It involves an imbalance of reproductive hormones (androgens and insulin).
Yes, many endocrine disorders, such as Type 2 Diabetes and Thyroid disease, run in families. If your parents have them, you have a higher risk.
Most endocrine disorders are chronic, meaning they cannot be “cured” permanently, but they can be successfully managed. With the right medication and lifestyle changes, you can live a completely normal, healthy life.
The most common culprits for unexplained weight gain are Hypothyroidism (underactive thyroid), PCOS, Cushing’s Syndrome (excess cortisol), and Insulin Resistance.
బ్లాస్టోసిస్ట్ కల్చర్, గర్భాశయానికి విజయవంతంగా అతుక్కునే (ఇంప్లాంటేషన్) అత్యధిక సామర్థ్యం ఉన్న పిండాలను గుర్తించడానికి వీలు కల్పిస్తుంది. ఈ పద్ధతి, బదిలీ చేసే పిండాల సంఖ్యను తగ్గించడానికి సహాయపడుతుంది, తద్వారా కవలలు లేదా అంతకంటే ఎక్కువ మంది పిల్లలు పుట్టే అవకాశాన్ని తగ్గిస్తుంది.
బ్లాస్టోసిస్ట్ కల్చర్లో, ఫలదీకరణం చెందిన అండాలను లేదా పిండాలను, వాటి పెరుగుదలకు అవసరమైన పోషకాలను అందించే ఒక ప్రత్యేకమైన కల్చర్ మీడియా (పోషక ద్రవం)లో ఉంచి పెంచుతారు. ఆ తర్వాత ఈ పిండాలను, మానవ శరీరం లోపలి పరిస్థితులను అనుకరించే ఇంక్యుబేటర్లో ఉంచి, వాటి అభివృద్ధికి తోడ్పడతారు.
బ్లాస్టోసిస్ట్ బదిలీ కోసం పిండాలను మరో 3 రోజులు అదనంగా ల్యాబ్లో పెంచాల్సి ఉంటుంది. అయినప్పటికీ, IVF ప్రక్రియలో ఈ అదనపు సమయం ఒక విలువైన పెట్టుబడిగా పరిగణించబడుతుంది. ఎందుకంటే, ఈ ఎక్కువ రోజుల పెంపకం, మరింత కచ్చితమైన పిండాల ఎంపికకు వీలు కల్పిస్తుంది, తద్వారా సక్సెస్ రేట్లు గణనీయంగా మెరుగుపడతాయి.
పిండాలు బ్లాస్టోసిస్ట్ దశకు అభివృద్ధి చెందడాన్ని అనేక అంశాలు ప్రభావితం చేస్తాయి. పరిశోధనల ప్రకారం, స్త్రీ వయసు తక్కువగా ఉండటం, గతంలో ఎక్కువసార్లు గర్భం దాల్చిన అనుభవం (higher parity), సాధారణ ఇన్సెమినేషన్ పద్ధతులు, మరియు తక్కువ మోతాదులో గోనడోట్రోపిన్లు (మందులు) వాడటం వంటి అంశాలు అధిక నాణ్యత గల బ్లాస్టోసిస్ట్లు ఏర్పడటానికి గణనీయంగా దోహదపడతాయని తేలింది.
Genital TB itself is not typically spread through sexual contact. However, if the patient has active lung TB, they can spread the infection through coughing or sneezing.
Yes, the bacterial infection can be completely cured with a 6 to 9-month course of antibiotics. However, the scarring or structural damage caused by the infection may be permanent.
It is a leading cause of infertility, but not every case results in permanent sterility. Early detection significantly improves the chances of preserving fertility.
Yes. Once the infection is cleared, you can plan for pregnancy. If your fallopian tubes are damaged, IVF is a highly successful option to help you conceive.
The standard course of Anti-Tubercular Therapy (ATT) usually lasts for 6 months, but depending on the severity, your doctor may extend it to 9 months.
Most home pregnancy tests can detect pregnancy within 7–10 days after a missed period. For the most accurate result, it’s best to test one week after your missed period. A blood test at a doctor’s clinic can confirm pregnancy even earlier.
Cramps without a period and a negative test could be due to late ovulation, stress, hormonal changes, PCOS, or lifestyle factors. If symptoms persist, consult a doctor to rule out pregnancy or other underlying conditions.
If your test is negative but your period hasn’t arrived, it could be due to late ovulation, stress, sudden weight changes, thyroid issues, or PCOS. Retest after a few days or see a doctor for further evaluation.
Yes, it’s possible. hCG levels (pregnancy hormone) rise rapidly in early pregnancy. Testing too early may give a false negative. Retesting after 2–3 days is recommended for more accurate results.
Yes, there’s still a chance. Some women produce lower levels of hCG that take longer to show up on home tests. A blood test or ultrasound can give a more reliable answer.
Common causes include: Stress or anxiety, Hormonal imbalance, Polycystic ovary syndrome (PCOS), Thyroid issues, Excessive exercise or sudden weight changes, or Perimenopause. If your period is more than 2–3 weeks late, consult a gynecologist.
The uterus naturally cleanses itself after a period by shedding the uterine lining. You don’t need to do anything special. Supporting your body with hydration, a balanced diet, and rest helps the natural process.
During menstruation, the body naturally expels blood and tissue. You should not try to “clean” the uterus directly. Instead, maintain good menstrual hygiene, change sanitary products regularly, and drink plenty of water to support your cycle.
Certain foods support uterine and reproductive health, including:
Leafy greens (spinach, kale – rich in iron)
Fruits (papaya, pineapple – help with blood flow)
Whole grains & legumes (fiber-rich for hormone balance)
Herbal teas (ginger, chamomile – reduce inflammation)
Omega-3 rich foods (flaxseeds, walnuts, fish – support circulation)
You don’t need external products or douching, as the womb cleans itself naturally. The best approach is healthy nutrition, hydration, and exercise to improve blood circulation and hormone balance.
While the womb naturally maintains itself, some signs of imbalance include Irregular or missed periods, Severe cramps or heavy bleeding, Unusual discharge or odor, Pelvic pain, and Fertility issues. If these occur, consult a gynecologist instead of trying DIY cleansing.
అప్పుడప్పుడు వాడితే పెద్దగా ఇబ్బంది ఉండదు. కానీ, తరచుగా లేదా దీర్ఘకాలం పాటు కొన్ని రకాల రసాయన లూబ్రికెంట్స్ వాడితే సమస్యలు రావచ్చు. ఇవి ఇన్ఫెక్షన్లకు లేదా హార్మోన్ల మార్పులకు కారణం కావచ్చు. మీకు సందేహం ఉంటే డాక్టర్ని అడగడం మంచిది.
అవును. యోనిలో pH విలువ 3.8 నుండి 4.5 మధ్య ఉంటేనే అది ఆరోగ్యకరమైన వాతావరణం. ఇది ఇన్ఫెక్షన్ల నుండి కాపాడుతుంది మరియు వీర్య కణాలు బతకడానికి సహాయపడుతుంది. చాలా లూబ్రికెంట్స్ ఈ pH ను మార్చేస్తాయి, తద్వారా గర్భం దాల్చడం కష్టమవుతుంది. అందుకే pH-బ్యాలెన్స్డ్ (pH-balanced) లూబ్రికెంట్స్ మాత్రమే వాడాలి.
ఈ చికిత్సల సమయంలో సాధారణ లూబ్రికెంట్స్ వాడకపోవడమే మంచిది. ఎందుకంటే అవి వీర్య కణాల పనితీరును లేదా పిండం ఎదుగుదలను దెబ్బతీయవచ్చు. మీ ఫెర్టిలిటీ స్పెషలిస్ట్ సలహా మేరకు మాత్రమే వాడాలి.
వద్దు. ఆయిల్ ఆధారిత (Oil-based) లూబ్రికెంట్స్ వీర్య కణాలను దెబ్బతీస్తాయి మరియు ఇన్ఫెక్షన్ల రిస్క్ను పెంచుతాయి. నీటి ఆధారిత (Water-based) లేదా ప్రత్యేకంగా గర్భధారణ కోసం రూపొందించిన (Fertility-friendly) లూబ్రికెంట్స్ వాడటమే సురక్షితం.
జన్యుపరమైన లోపాలు, ఇన్ఫెక్షన్లు, వెరికోసిల్ (Varicocele), ధూమపానం, మద్యపానం, అధిక ఒత్తిడి మరియు రసాయనాలకు గురవడం ప్రధాన కారణాలు.
అవును. జీవనశైలి మార్పుల ద్వారా దీనిని సరిచేసుకోవచ్చు. ఒకవేళ తీవ్రమైన సమస్య ఉంటే IUI, IVF లేదా ICSI చికిత్సల ద్వారా 15-70% సక్సెస్ పొందవచ్చు.
WHO 2025 ప్రమాణాల ప్రకారం… కనీసం 4% నుండి 14% వీర్య కణాలు సరైన ఆకారంలో ఉంటే అది ఆరోగ్యకరమైన స్థితి
వీర్య కణాల సైజు మరియు ఆకారాన్ని (Size and Shape) ‘స్పెర్మ్ మార్ఫాలజీ’ అంటారు. కేవలం 4% కణాలు బాగున్నా సరే, సహజంగా పిల్లలు పుట్టే అవకాశం ఉంటుంది.
అవును. ఘాటైన రసాయనాలు (Chemicals), పెర్ఫ్యూమ్స్ మరియు కాలుష్యం embryo /పిండంపై ప్రభావం చూపవచ్చు. సాధ్యమైనంత వరకు స్వచ్ఛమైన గాలిలో, ప్రశాంతమైన వాతావరణంలో ఉండటానికి ప్రయత్నించండి.
వద్దు. కొన్ని రకాల హెర్బల్ టీలలో కెఫిన్ లేదా ఇతర పదార్థాలు ఉండవచ్చు. అవి మీరు వాడుతున్న మందులతో రియాక్ట్ అయ్యే ప్రమాదం ఉంది. మీ డాక్టర్ చెబితే తప్ప, సొంతంగా హెర్బల్ మందులు వాడకండి.
గర్భం కచ్చితంగా నిలుస్తుందని గ్యారెంటీ ఇచ్చే పని ఏదీ లేదు. కానీ… మంచి పోషకాహారం తీసుకోవడం, టైంకి మందులు వేసుకోవడం (ముఖ్యంగా ఫోలిక్ యాసిడ్), ఒత్తిడి లేకుండా ఉండటం మరియు వేడికి దూరంగా ఉండటం వల్ల సక్సెస్ రేట్ పెరుగుతుంది.
చేయవచ్చు. ట్రాన్స్ఫర్ తర్వాత ప్రయాణం చేయడం వల్ల పిండం పడిపోతుందని చెప్పడానికి శాస్త్రీయ ఆధారాలేవీ లేవు. అయితే, కుదుపులు లేని ప్రయాణం చేయడం మరియు ఒత్తిడి లేకుండా చూసుకోవడం ముఖ్యం.
ట్రాన్స్ఫర్ తర్వాత బెడ్ రెస్ట్ అవసరం లేదు, కానీ మొదటి రెండు వారాలు (టెస్ట్ రిజల్ట్ వచ్చే వరకు) రిలాక్స్గా ఉండటం మంచిది. వంట చేసుకోవడం, నడవటం వంటి చిన్న పనులు చేసుకోవచ్చు కానీ బరువులు ఎత్తడం, పరిగెత్తడం వంటివి చేయకూడదు.
ముందుగా డాక్టర్ని కలిసి పూర్తి ఆరోగ్య పరీక్షలు చేయించుకోవాలి. మీ శరీర తత్వాన్ని బట్టి, డాక్టర్లు సూచించిన ఆహార మార్పులను పాటించాలి. ఈ ప్రక్రియలో మీ డాక్టర్తో ఎప్పటికప్పుడు మాట్లాడుతూ ఉండాలి.
కేవలం ఆహారమే కాకుండా, జీవనశైలి మార్పులు (Lifestyle modifications) మరియు అవసరమైతే అధునాతన వైద్య చికిత్సలు (Advanced medical interventions) కూడా ఉంటాయి. మీ సమస్య తీవ్రతను బట్టి డాక్టర్లు IUI లేదా IVF వంటి చికిత్సలను సూచిస్తారు.
మీకు నెలసరి సరిగ్గా రాకపోయినా (Irregular periods), పెళ్ళై ఏడాది దాటినా పిల్లలు పుట్టకపోయినా లేదా ఇతర ఆరోగ్య సమస్యలు ఉన్నా వెంటనే డాక్టర్ని కలవాలి. ఎంత త్వరగా సమస్యను గుర్తిస్తే, చికిత్స అంత సులభమవుతుంది.
కచ్చితంగా. మంచి పోషకాహారం తీసుకోవడం వల్ల అండం మరియు వీర్య కణాల నాణ్యత పెరుగుతుంది. ఇది వయసు మరియు ఇతర ఆరోగ్య పరిస్థితులపై కూడా ఆధారపడి ఉంటుంది. స్పెషలిస్ట్ డాక్టర్లు మీ కండిషన్ను బట్టి ఆహార ప్రణాళికను రూపొందిస్తారు.
సంతానలేమి అనేది కేవలం ఒకరి సమస్య కాదు. సరైన ఆహారం, వ్యాయామం మరియు వైద్యుల పర్యవేక్షణ ద్వారా దీనిని జయించవచ్చు. మీ వ్యక్తిగత సమస్యలను బట్టి సరైన చికిత్సను ఎంచుకోవడం ముఖ్యం.
అవును, కచ్చితంగా ఉపయోగపడతాయి. చికిత్స సమయంలో ఉండే భయం, ఆందోళనను తగ్గించుకోవడానికి ఇవి సహాయపడతాయి. అయితే, అక్కడ నేర్పించే ఆసనాలు మీ చికిత్సకు ఇబ్బంది కలగనివ్వకుండా ఉన్నాయా లేదా అని మీ డాక్టర్ని అడిగి తెలుసుకోవాలి.
పిల్లలు కలగకపోవడం వల్ల వచ్చే బాధ, ఒత్తిడిని తట్టుకోవడానికి ధ్యానం (Meditation) మరియు ప్రాణాయామం (Breathwork) బాగా పనిచేస్తాయి. మనసును ప్రశాంతంగా ఉంచుకుని, పాజిటివ్గా ఆలోచించే శక్తిని ఇవి ఇస్తాయి.
సాధారణంగా యోగా చేసేటప్పుడు సాత్విక ఆహారం మంచిది. ఆకుకూరలు, పండ్లు, ఆరోగ్యకరమైన కొవ్వులు మరియు యాంటీఆక్సిడెంట్స్ ఎక్కువగా ఉండే ఆహారం తీసుకోవాలి. హార్మోన్లకు ఇబ్బంది కలిగించే ఆహారాలకు దూరంగా ఉండాలి.
అవును. ఫెర్టిలిటీ యోగా ఇద్దరికీ మంచిదే.
- స్త్రీలకు: అండాశయాల ఆరోగ్యం మరియు హార్మోన్ల సమతుల్యత కోసం.
- పురుషులకు: వీర్య కణాల ఆరోగ్యం మరియు ఒత్తిడి తగ్గడం కోసం. ఇద్దరూ కలిసి యోగా చేయడం వల్ల బంధం బలపడుతుంది, ఆరోగ్యం మెరుగుపడుతుంది.
Yes. Around weeks 4-6, implantation bleeding or hormonal shifts often cause light pink blood during early pregnancy (spotting). It is very common.
Stress itself doesn’t cause bleeding, but high stress can lead to cramping or muscle tension. It is best to stay calm and rest.
Yes. Doctors usually recommend avoiding strenuous activity and sexual intercourse for a few days until the pink spotting resolves during pregnancy.
Yes. The cervix is very sensitive and engorged with blood. Friction during sex can cause tiny capillaries to burst, resulting in pink discharge.
Call your doctor if:
- The discharge turns bright red.
- You soak a pad.
- You feel severe abdominal pain or dizziness.
- You pass clots/tissue.